The ACROSTART study was intended to determine the time to achieve normalization of GH and IGF-I levels in responding patients with acromegaly administered different dosage regimens of lanreotide Autogel (Somatuline® Autogel®).
MethodsFrom March 2013 to October 2013, clinical data from 57 patients from 17 Spanish hospitals with active acromegaly treated with lanreotide for ≥4 months who achieved hormonal control (GH levels <2.5ng/ml and/or normalized IGF-I levels in ≥2 measurements) were analyzed. The primary objective was to determine the time from start of lanreotide treatment to hormonal normalization.
ResultsMedian patient age was 64 years, 21 patients were male, 39 patients had undergone surgery, and 14 patients had received radiotherapy. Median hormonal values at start of lanreotide treatment were: GH, 2.6ng/ml; IGF-I, 1.6×ULN. The most common starting dose of lanreotide was 120mg (29 patients). The main initial regimens were 60mg/4 weeks (n=13), 90mg/4 weeks (n=6), 120mg/4 weeks (n=13), 120mg/6 weeks (n=6), and 120mg/8 weeks (n=9). An initial treatment regimen with a long interval (≥6 weeks) was administered in 25 patients. Mean duration of lanreotide treatment was 68 months (7–205). Median time to achieve hormonal control was 4.9 months. Injections were managed without healthcare assistance in 13 patients. Median number of visits to endocrinologists until hormonal control was achieved was 3. Fifty-one patients were “satisfied”/“very satisfied” with treatment and 49 patients did not miss any dose.
ConclusionsReal-life treatment with lanreotide Autogel resulted in early hormonal control in responding patients, with high treatment adherence and satisfaction despite disparity in starting doses and dosing intervals.
El objetivo del estudio ACROSTART era determinar el período de tiempo para lograr la normalización hormonal (GH e IGF-I) en pacientes con acromegalia respondedores al tratamiento considerando los regímenes de lanreótida Autogel (Somatuline® Autogel®) utilizados en la práctica clínica.
MétodosDesde marzo de 2013 hasta octubre de 2013, en 17 hospitales españoles se analizaron los datos clínicos de 57 pacientes con acromegalia activa tratados con lanreótida durante ≥4 meses que lograron control hormonal (niveles de GH <2,5ng/ml y/o IGF-I normalizado en ≥2 evaluaciones). El objetivo principal fue determinar el período de tiempo desde el inicio del tratamiento con lanreótida hasta la normalización hormonal.
ResultadosLa mediana de edad de los pacientes fue 64 años, 21 pacientes eran hombres, 39 pacientes habían recibido cirugía, 14 pacientes habían recibido radioterapia. Los valores hormonales medianos al inicio del tratamiento con lanreótida fueron GH: 2,6ng/ml, IGF-I: 1,6×LSN. La dosis inicial más frecuente de lanreótida fue de 120mg (29 pacientes). Los principales regímenes iniciales fueron 60mg/4 semanas (n=13), 90mg/4 semanas (n=6), 120mg/4 semanas (n=13), 120mg/6 semanas (n=6), 120mg/8 semanas (n=9). Se administró un régimen de intervalo prolongado (≥6 semanas) en 25 pacientes. La duración media del tratamiento con lanreótida fue de 68 meses (7–205). El tiempo medio hasta lograr el control hormonal fue de 4,9 meses. Las inyecciones se manejaron sin asistencia médica en 13 pacientes. La mediana del número de visitas al endocrinólogo hasta el control hormonal fue 3. Cincuenta y un pacientes estaban “satisfechos”/“muy satisfechos” con el tratamiento y 49 pacientes no olvidaron ninguna dosis.
ConclusionesEl tratamiento en la vida real con lanreótida Autogel condujo a un control hormonal temprano en pacientes que respondieron, con una alta adherencia al tratamiento y satisfacción con el tratamiento, a pesar de la disparidad de las dosis iniciales y los intervalos de dosificación.
Acromegaly is a rare chronic endocrine disease, characterized by enhanced growth hormone (GH) secretion and elevated insulin-like growth factor-I (IGF-I) levels; the most frequent cause of which is a benign pituitary adenoma.1 Persistently high levels of GH and IGF-I cause significant mortality and morbidity, mainly due to cardiovascular and respiratory complications.2 Therefore, control of GH and IGF-I secretion is decisive in improving survival.3
The current goals of acromegaly treatment are to eliminate the tumor or, if not possible, to reduce or control its growth, normalization of GH and IGF-I levels and prevention and adequate management of comorbidities.2 Therapeutic management strategies include surgery, radiotherapy and medical treatment. Pharmacological therapy plays a key role in managing patients with acromegaly when surgery or radiotherapy are not an option, and consists of somatostatin analogs (SSAs), lanreotide, octreotide, and pasireotide, as well as dopamine agonists and GH receptor antagonists. SSAs have become the pillar of acromegaly medical therapy in patients who are unsuitable for, or refuse surgery, after failure of surgical treatment, or as primary treatment in selected cases.4
Initial recommended doses of the SSA lanreotide Autogel (Somatuline® Autogel®, Ipsen Pharma) are 60, 90 and 120mg administered every 28 days. The long-term safety and efficacy of lanreotide Autogel in patients with acromegaly has been analyzed in previous studies. In a large cohort of acromegalic patients, sustained control of GH and IGF-I levels was obtained with lanreotide Autogel, with approximately 50% of patients obtaining GH levels <2.5ng/ml and/or normalized IGF-I levels by week 16 of treatment.5 Experience in studies evaluating the use of lanreotide indicated that a longer dosage interval could be used in well controlled patients with similar efficacy,6–9 thus suggesting that extending the dosing interval to 42 and 56 days may be a common therapeutic strategy. The starting dose for lanreotide Autogel prescribed in Spain may differ from prescribing recommendations.8
In view of the above, this study (ACROSTART) was proposed to determine the time to achieve hormonal normalization control considering the initial doses and dosage intervals of lanreotide Autogel commonly used in clinical practice.
Material and methodsStudy designACROSTART was a multicenter, non-interventional, retrospective, post-authorization study performed in adult patients with active acromegaly conducted at 17 centers in Spain. The centers who participated in the study were experienced in the management of acromegaly. Responder patients whose acromegaly was managed with lanreotide Autogel were sequentially proposed from 08 March to 31 October 2013 to participate in the study. As this was a non-interventional study, the decision to prescribe lanreotide Autogel was made prior to and independently from the patient's enrolment into the study and it was prescribed as routinely at each center. The dosing regimens to be administered were entirely at the discretion of the treating physician.
Study included adults (≥18 years) with active acromegaly (GH levels ≥2.5ng/ml and/or non-normalized IGF-I levels) who had been treated with lanreotide Autogel monotherapy for at least 4 months and achieved GH levels <2.5ng/ml and/or normalized IGF-I levels on at least two consecutive evaluations (responder patients). The measurements must have been done with at least a 4-month interval prior to the study. Patients whose clinical records lacked information regarding the date and initial dose of lanreotide Autogel and the first hormonal response available were excluded. Patients that did not achieve hormone control or achieved only partial hormonal control were excluded from the study. The study included both patients who had undergone prior surgery or radiotherapy or who were treatment naive. Patients who had received prior treatment with another SSA or other acromegaly drugs were also allowed to be included in the study. All patients provided written informed consent.
To guarantee the retrospective and observational nature of the study, the information was collected retrospectively from the patient's clinical history chart and no additional diagnostic or therapeutic interventions were performed. Approval was obtained from the appropriate regulatory bodies, prior to study initiation. This study adhered to all local regulatory requirements applicable to non-interventional studies. Before initiating the study, each investigator/institution had obtained written and dated approval/favorable opinion from the Independent Ethics Committee (IEC)/Institutional Review Board (IRB). The study was performed in accordance with the requirements expressed in the Declaration of Helsinki, as well as Spanish legislation concerning observational studies. This study was funded by Ipsen Pharma S.A., Spain.
AssessmentsThe following data were collected from patients’ clinical history, when available: sociodemographic and socio-familiar data, data on the diagnosis of acromegaly, data related to the treatment of acromegaly, changes in co-morbidities (diabetes, carpal tunnel syndrome, sleep apnea and arterial hypertension) and concomitant medications for arterial hypertension and diabetes, data related to tumor control. Data related to patient satisfaction (at the time that the patient provided informed signed consent, the investigator asked a direct question to the patient about satisfaction with 3 possible answers [Very satisfied, satisfied, not at all satisfied]) and adherence to treatment (determined by the number of omitted doses and by patients’ continuation of the treatment at the end of the study), and clinical and economic aspects (use of healthcare resources: endocrinologist and nurses visits, laboratory and image testing, days of absenteeism [days of absenteeism were defined as the total sum of visits to doctor, hospital nurse or outpatient nurse. Each visit was considered as one day of absenteeism]) until obtaining control of the disease.
The levels of GH and IGF-I were noted at the following times: at diagnosis, presurgically, after surgical treatment, start of treatment with lanreotide Autogel and when these levels were normalized during treatment with lanreotide Autogel. As this is a retrospective, non-interventional study, hormone levels were measured as per clinical practice at each center; the methods were not standardized across different centers. In addition, the frequency of study visits, biochemical testing, and radiological evaluation as well as the methods used were determined by the endocrinologist in charge.
As this was a non-interventional study in which lanreotide Autogel was to be administered and managed within routine medical care, adverse event (AE) reporting followed regulations related to spontaneous cases. Investigators were asked to report only related AEs (non serious and serious) to the safety department of the manufacturer of lanreotide Autogel if this had not been already done at the time of onset of the AE, using the usual process for such reactions.
ObjectivesThe primary objective was to determine the time to achieve hormonal control (defined as GH levels <2.5ng/ml and/or normalized IGF-I on at least 2 different evaluations) considering the starting dose and dosage intervals of lanreotide Autogel.
Secondary objectives included collecting clinical data on patients according to initial treatment regimen, including changes in co-morbidities, determining the ability of the self-inject either by the patient or a relative, the use of healthcare resources until obtaining hormonal control, assessing the effectiveness of lanreotide Autogel in controlling tumor size, evaluating patients’ general satisfaction with lanreotide Autogel therapy, and assessing patients’ adherence to lanreotide Autogel therapy.
Sample sizeThe sample size calculation was based on the number of patients that, when included in the study, would provide sufficient information to determine the primary study objective. The long-term safety and efficacy of lanreotide Autogel in patients with acromegaly has been analyzed in previous studies. Specifically, the study by Melmed et al.5 in a large cohort of unselected patients showed sustained control of GH and IGF-I levels with lanreotide Autogel, with approximately 50% of patients obtaining GH levels <2.5ng/ml and/or normalized IGF-I levels in week 16 of treatment with the following doses: 60, 90, 120mg every 28 days. This level of control was also observed with an injection frequency every 8 weeks.8 Thus, the sample size was calculated so that the group of patients included in the ACROSTART study permitted an estimation of an assumed stabilization of 50% of patients at 16 weeks, with a precision of ±0.14 percentage units. Assuming a 2-sided alpha risk of 0.05 (type I error), and a percentage of loss due to incomplete data, inconsistencies, etc. of no more than 20%, a total of 59 patients were to be included.
Statistical analysisThe primary analysis set (PAS) consisted of all screened patients who had taken lanreotide Autogel for at least 4 months, with date of hormone normalization recorded and without major protocol deviations. The primary and secondary objectives analyses were performed on the PAS population. The primary objective was the time to hormonal normalization on at least 2 different evaluations considering the commonly used starting doses and dosage intervals of lanreotide Autogel. Hormonal control was considered to have been achieved at the second evaluation and this time point was used for statistical analyses. The primary and secondary endpoints were reported using descriptive quantitative summary statistics. To determine the effectiveness of lanreotide Autogel in controlling tumor size, descriptive statistics of tumor volume and percentage tumor shrinkage were obtained, as follows:
% tumor shrinkage (at the most recent evaluations during treatment with lanreotide Autogel with regards to the start of treatment)=[(Maximum tumor volume at the most recent evaluations during treatment with lanreotide Autogel−Maximum tumor volume at the start of treatment)/(Maximum tumor volume at the start of treatment)]*100.
ResultsPatientsSixty-two patients were screened from 08 March to 31 October 2013. Five patients were excluded from the analysis because they underwent surgery or received radiotherapy after the initiation of treatment with lanreotide Autogel but before achieving hormonal control. The PAS population consisted of 57 patients with a median age of 64 years (range 23–90) at study entry and a median age at diagnosis of 51 years (range 13–77). Demographic characteristics are shown in Table 1. At study entry there were 5 patients ≥80 years old (80 years n=1, diagnosed at 53 years with extrasellar macroadenoma; 83 years n=2, both diagnosed at 65 years, both with microadenoma; 88 years n=1, diagnosed at 77 years with microadenoma; and 90 years n=1, diagnosed at 72 years with intrasellar macroadenoma); 4 were females.
Patient demographics and disease characteristics.
| PASN=57 | 60mg/4 weeksn=13 | 90mg/4 weeksn=6 | 120mg/4 weeksn=13 | 120mg/6 weeksn=6 | 120mg/8 weeksn=9 | |
|---|---|---|---|---|---|---|
| Male, n (%) | 21 (36.8) | 4 (30.8) | 1 (16.7) | 6 (46.2) | 3 (50.0) | 3 (33.3) |
| Patient able to self-inject, n (%) | 8 (14.0) | 2 (15.4) | 2 (33.3) | 2 (15.4) | 0 | 0 |
| Injection by family member/relative, n (%) | 5 (8.8) | 1 (7.7) | 0 | 1 (7.7) | 1 (16.7) | 0 |
| Injection by healthcare staff, n (%) | 44 (77.2) | 10 (76.9) | 4 (66.7) | 10 (76.9) | 5 (83.3) | 9 (100) |
| Median age, years (range) | 64 (23–90) | 67 (27–83) | 51.5 (30–77) | 57 (32–88) | 73 (23–90) | 70 (37–83) |
| Median time since diagnosis until study entry, months (range) | 103.5 (8.1–325.5) | 100.7 (14.6–285.0) | 103.7 (8.1–128.4) | 129.9 (19.3–284.2) | 170.1 (17.9–325.5) | 120.8 (41.6–218.6) |
| Type of adenoma, n (%) | ||||||
| Microadenoma | 22 (38.6) | 8 (61.5) | 2 (33.3) | 6 (46.2) | 0 | 2 (22.2) |
| Macroadenoma | 35 (61.4) | 5 (38.5) | 4 (66.7) | 7 (53.8) | 6 (100) | 7 (77.8) |
| Prior treatment, n (%) | ||||||
| Surgery | 39 (68.4) | 8 (61.5) | 5 (83.3) | 7 (53.8) | 4 (66.7) | 7 (77.8) |
| Radiotherapy | 14 (24.6) | 2 (15.4) | 2 (33.3) | 6 (46.2) | 1 (16.7) | 1 (11.1) |
| Hormonal values at start of lanreotide Autogel treatment | ||||||
| GH, ng/ml, median (range) | 2.6 (0.2–48.2) | 2.65 (0.2–20.5) | 3.90 (3.2–48.2) | 2.52 (1.1–37.5) | 1.58 (0.5–3.7) | 2.84 (0.9–8.0) |
| IGF-I (×ULN), median (range) | 1.6 (1.0–4.4) | 1.6 (1.1–3.2) | 4.0 (1.1–4.4) | 1.4 (1.0–3.4) | 1.1 (1.0–1.9) | 1.4 (1.0–2.2) |
Microadenoma ≤10mm, macroadenoma >10mm.
PAS, primary analysis set; ULN, upper limit of normal.
Most patients (68.4%) began treatment because they were not able to obtain adequate disease control with surgery. The median time since the last surgery to start treatment with lanreotide Autogel was 33.9 months (range, 2.3–191.0 months). There were 5 (12.8%) patients that had more than one surgery. Presurgery and post-surgery hormone data are found in Table 2. There were 14 patients (24.6%) that underwent radiotherapy. The median time since the last radiotherapy to start treatment with lanreotide Autogel was 12.6 months (range, 3.3–147.6 months). There were 11 (19.3%) patients that underwent both surgery and radiotherapy, 28 (49.1%) that underwent only surgery, 3 (5.3%) that underwent only radiotherapy, and 15 (26.3%) patients that had neither prior surgery or radiotherapy.
Hormone data in the PAS population (N=57).
| At diagnosis | Following presurgical treatment | After surgery | At start of lanreotide Autogel treatment | At hormone controlb | |
|---|---|---|---|---|---|
| GH (ng/ml), na | 54 | 16 | 35 | 53 | 46 |
| Median (range) | 8.0 (0.2–177.0) | 2.0 (0.2–63.3) | 3.1 (0.1–177.0) | 2.6 (0.2–48.2) | 0.83 (0.1–5.7) |
| IGF-I (×ULN), na | 40 | 14 | 32 | 53 | 55 |
| Median (range) | 2.2 (0.9–5.1) | 1.4 (0.7–5.2) | 1.4 (0.2–7.4) | 1.6 (1.0–4.4) | 0.8 (0.4–1.5) |
GH, growth hormone; IGF, insulin growth factor; PAS, primary analysis set; ULN, upper limit of normal.
Injections were managed without assistance of healthcare staff in 22.8% of the patients, either with self-injections or administration by close relatives. A total of 19 (33.3%) patients received pre-surgical treatment. The most frequent pre-surgical treatment was lanreotide by 10 (17.5%) patients, followed by octreotide by 6 (12.3%) patients, and cabergoline by 3 (5.3%) patients.
When treatment with lanreotide Autogel was started, 18 patients were receiving pharmacotherapy for acromegaly, 10 (17.5%) with SSAs (4 lanreotide SR, 4 octreotide LAR, and 2 octreotide), 7 (12.3%) with dopamine agonists, 1 (1.8%) with GH receptor antagonists. At initiation of treatment with lanreotide Autogel, median hormonal values were GH: 2.6ng/ml (range 0.2–48.2) and IGF-I: 1.6×ULN (range 1.0–4.4); 29 patients had GH levels ≥2.5ng/ml (47 patients ≥1ng/ml) and all patients had non-normalized IGF-I levels.
Concerning signs and symptoms of acromegaly at diagnosis, acral growth was the most frequent, reported by 51 (89.5%) patients, followed by headache in 35 (61.4%) patients and arthralgia in 27 (47.4%) patients. Median hormonal values at start of lanreotide treatment were GH: 2.6ng/ml (range 0.2–48.2) and IGF-I: 1.6×ULN (range 1.0–4.4).
Mean treatment duration with lanreotide Autogel was 68 months (95% CI 55–80). At the end of the study period, 47 (82.5%) patients continued with active treatment. Lanreotide Autogel 120mg was the most common starting dose (29 [51%] patients). An extended dosing interval (6, 8 and 12 weeks) at lanreotide Autogel initiation was reported in 25 (44%) patients included in the study. Five different subgroups were further analyzed according to starting dosage pattern: 60mg/4 weeks (n=13), 90mg/4 weeks (n=6), 120mg/4 weeks (n=13), 120mg/6 weeks (n=6), and 120mg/8 weeks (n=9). Other dosages included: 90mg/6 weeks (n=4), 90mg/8 weeks (n=3), 60mg/8 weeks (n=2) and 120mg/12 weeks (n=1). Due to the small number of patients, these subgroups were not analyzed separately (Table S1).
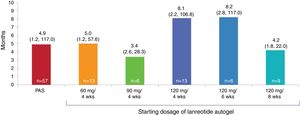
Primary endpointThe median time to reach hormonal control was 4.9 months (range 1.2–117.0), Fig. 1. The median time to reach hormonal normalization in the 120mg/6 weeks subgroup (n=6) was 8.2 months (range 2.8–117.0), in the 120mg/4 weeks subgroup (n=13) was 8.1 months (range 2.2–106.8), in the 60mg/4 weeks subgroup (n=13) was 5.0 months (range 1.2–57.6), in the 120mg/8weeks subgroup (n=9) was 4.2 months (range 1.8–22.0), and in the 90mg/4 weeks subgroup (n=6) was 3.4 months (range 2.6–28.3). More than half of the patients (n=37, 64.9%) achieved hormonal control in less than 10 months; however, there were 4 patients that took over 3 years to reach hormonal control (Table S2). The median time to reach hormonal control among the 47 patients who were not receiving an SSA when starting treatment with lanreotide Autogel was similar to the overall population (4 months [range, 1–107 months]).
Median time to hormonal control, months (range). The 10 patients with other starting dosages of lanreotide Autogel: 90mg/6 weeks (n=4), 90mg/8 weeks (n=3), 60mg/8 weeks (n=2) and 120mg/12 weeks (n=1), have not been analyzed separately due to the small number of patients in each subgroup. PAS, primary analysis set.
Median hormonal values when hormonal control was achieved were GH: 0.83ng/ml (range 0.1–5.7) and IGF-I: 0.8×ULN (range 0.4–1.5) (Table 2). The median GH and IGF-I values in each dosage subgroup were 0.80ng/ml (range 0.1–4.3) and 0.9xULN (range 0.4–1.5) in the 60mg/4 weeks subgroup, 0.50ng/ml (range 0.3–2.0) and 0.7×ULN (range 0.6–1.4) in the 90mg/4 weeks subgroup, 1.25ng/ml (range 0.2–5.7) and 0.8×ULN (range 0.4–1.0) in the 120mg/4 weeks subgroup, 1.93ng/ml (range 0.7–4.5) and 0.8×ULN (range 0.6–1.0) in the 120mg/6 weeks subgroup, and 0.99ng/ml (range 0.4–2.8) and 0.8×ULN (range 0.5–1.0) in the 120mg/8 weeks subgroup.
Secondary endpointsUse of healthcare resourcesGlobally, from the start of treatment until hormone control obtained, the median number of endocrinologist visits was 3 (range 1–64), of hospital nurse visits was 1 (range 0–64), and outpatient nurse visits was 1 (range 0–132).
Overall, the median number of laboratory tests performed was 2.5 (range 1–20) while the median number of imaging tests was 1 (range 0–9). The median number of days of absenteeism overall was 6.5 (range 2–151) days until hormone control was obtained. For the different subgroups, the median number of days of absenteeism was 10 (range 3–151) in the 60mg/4 weeks subgroup, 8.5 (range 5–20) in the 90mg/4 weeks subgroup, 13 (range 3–146) in the 120mg/4 weeks subgroup, 9 (range 2–81) in the 120mg/6 weeks subgroup, and 6 (range 2–23) in the 120mg/8 weeks subgroup.
Control of co-morbiditiesHypertension was reported in 29 (51%) patients, both at the start of lanreotide Autogel treatment and when hormone control was achieved (28 patients had arterial hypertension at both time points and 1 patient was reported with arterial hypertension at the start of treatment, but not at hormone control and 1 patient did not have arterial hypertension at the start of treatment, but did at hormone control). The daily dose of medication for hypertension was maintained in 17 (60.7%) patients, the active principle was changed for 5 (17.9%) patients, daily dose was increased in 2 (7.1%) patients, medication was discontinued in 2 (7.1%) patients, daily dose was reduced in 1 (3.6%) patient, and the active principle was changed plus the daily dose was increased in 1 (3.6%) patient. Information on hypertension medication was missing in 1 patient.
At the start of treatment with lanreotide Autogel 11 (19.6%) patients had diabetes. When biochemical control of acromegaly was achieved, 16 (28.1%) patients were reported with diabetes and had a median HbA1c level of 6.9% (range 5.5–8.1). The daily dose of diabetes medication was maintained in 7 (50.0%) patients, increased in 3 (21.4%) patients, and reduced 2 (14.3%) patients, finally, the medication was changed in 2 (14.3%) patients. Information on diabetes medication was missing in 2 patients.
Carpal tunnel syndrome was reported in 5 (8.8%) patients when lanreotide Autogel treatment was started and when hormone control was reached, it was present in 2 patients (3.5%). Sleep apnea was reported at the start of treatment in 11 (19.3%) patients. When hormone control was reached, sleep apnea was reported in 12 (21.1%) patients. Ten patients of the patients with sleep apnea reported this co-morbidity at both time points (start of treatment and at hormone control). In 1 patient sleep apnea was reported at the start of treatment, but not at hormone control. Two patients did not report sleep apnea at the start of treatment but did at hormone control).
Tumor sizeAt diagnosis, 22 (38.6%) of patients had microadenoma and 35 (61.4%) had macroadenoma (21 with extrasellar extension and 14 intrasellar). There were 19 patients that had information on tumor volume at the beginning of treatment with lanreotide Autogel, the mean maximum tumor volume in these patients was 2010mm3 (standard deviation [SD] 7801mm3). Only 12 patients had information on tumor volume both at the beginning of lanreotide Autogel and at the last evaluation during treatment with lanreotide Autogel. The mean maximum tumor volume in these 12 patients was 3182mm3 (SD 38186mm3) at the start of treatment with lanreotide Autogel and was 2218mm3 (SD 26616mm3) at the most recent evaluation during treatment with lanreotide Autogel. In these 12 patients, there was a mean percentage of tumor reduction of 46.7% (SD 36.9%).
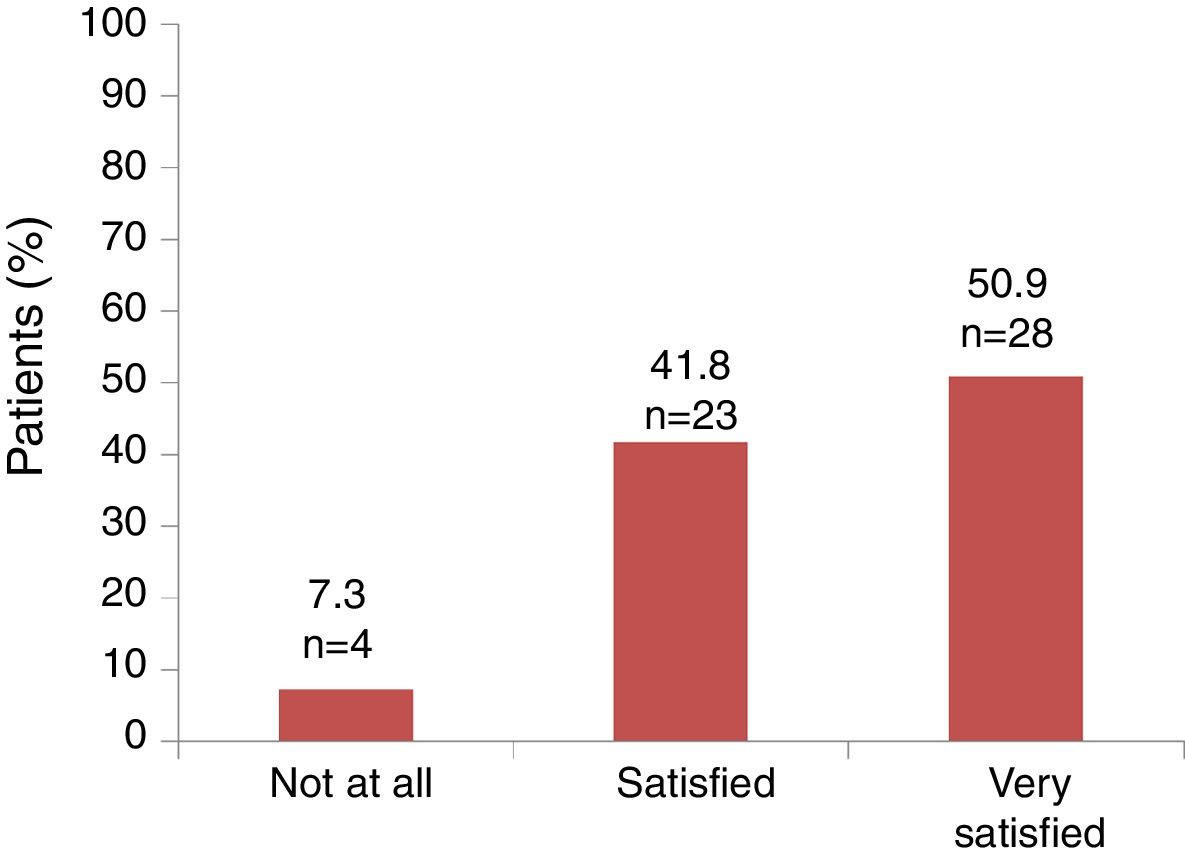
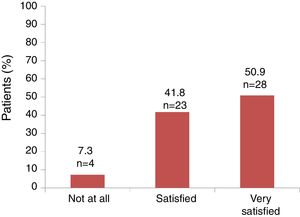
Satisfaction and adherenceGlobally, 51 out of 55 (92.7%) patients referred to be satisfied or very satisfied with the lanreotide Autogel treatment (Fig. 2). Only 4 (7.3%) patients were not at all satisfied with the treatment: one patient in the 90mg/4 weeks subgroup, one patient in the 120mg/4 weeks subgroup and 2 patients in the 120mg/8 weeks subgroup, respectively. Information on “satisfaction” was not available for 2 patients.
There was good adherence to therapy. Globally 49 (86.0%) patients declared not to miss any dose during the reporting period. The dose omissions in 8 patients were as follows: 1 dose omitted (n=2), 1 or 2 doses omitted (n=1), 2 doses omitted (n=3), 5 doses omitted (n=1), not specified (n=1). When subgroups were analyzed, compliance to treatment was observed in 92.3% patients in the 60mg/4 weeks subgroup, 83.3% patients in the 90mg/4 weeks subgroup, 84.6% patients in the 120mg/4 weeks subgroup, 66.7% patients in the 120mg/6 weeks subgroup and 77.8% for patients in the 120mg/8 weeks subgroup.
DiscussionIn a real-life setting, as analyzed in the ACROSTART study, the median time elapsed between initiating treatment with lanreotide Autogel and achieving hormonal control was 4.9 months. Real-life treatment with lanreotide Autogel led to a fast hormonal control, with a high treatment adherence and treatment satisfaction, despite disparity of starting doses and interval dosing. Very limited data have been published concerning the dosing of SSAs in routine clinical practice.6,7,10,11
In the ACROSTART study, there were 9 different starting dosing regimens in 57 patients, from the most (120mg/4 weeks) to the least (60mg/8 weeks) dose intensity, reflecting the disparity of treatment regimens in clinical practice. There did not appear to be a specific pattern explaining the investigator's choice of the initial dose and dosage interval of lanreotide Autogel. Patients might have started lanreotide Autogel treatment with an extended dosing interval if they were previously well controlled with octreotide LAR 20mg every 4 weeks (q4w) or 30mg q4w. However, patients who began with lower doses/prolonged dosing intervals of lanreotide Autogel might have had a shorter median time to hormone control achievement and may reflect a post-surgical population with lower initial IGF-I levels or smaller tumors. Additional analyses on patients that started treatment with lanreotide Autogel with an extended dose did not reveal any baseline characteristics that could explain the initial choice of treatment. Gender, age, time since diagnosis and hormonal values at start of treatment were similar. There were slightly more patients with macroadenomas in the group of patients starting with an extended dose 76% compared with 61% in the overall population.
SSAs have a well-established profile and patients do not typically discontinue treatment due to AEs.12 In patients with acromegaly, the approved starting dose for SSA therapy was determined in pharmacokinetic and pharmacodynamic studies. The initial dosage of lanreotide Autogel can be 60–120mg administered every 4 weeks followed by adjustment based on GH and/or IGF-I levels. Guidelines recommend increasing the SSA dose in response to the patient's need for additional symptom control or decreasing the dose if the patient has shown signs of improvement.2 The dose adjustments recommend maintaining 90mg/4 weeks if GH >1 to ≤2.5ng/ml, IGF-I normal and clinical symptoms controlled; increasing the dose to 120mg/4 weeks if GH >2.5ng/ml, IGF-I elevated and/or clinical symptoms uncontrolled; reducing the dose to 60mg q4w if GH ≤1ng/ml, IGF-I normal and clinical symptoms controlled; and considering an extended dosing interval of 120mg/6 or 8 weeks if patients are controlled on 60–90mg q4w. In the ACROSTART study, the main starting dosing regimens were 60mg/4 weeks and 120mg/4 weeks followed by 120mg/8 weeks. Based on published studies, dose titration of lanreotide appears to improve its efficacy in patients with acromegaly, with many patients requiring escalation to 120mg/4 weeks.13
Using pharmacokinetic model-based simulations, there was support that pharmacologically effective levels of lanreotide can be maintained after extending the dosing interval for lanreotide Autogel beyond the standard 4-week dosing interval.14 Studies evaluating alternative dosing schemes in patients with acromegaly that was well controlled, prolonging the time interval from 4 to 6/8 weeks between lanreotide Autogel injections did not lead to loss of efficacy.6,9,15,16
In the large international clinical trial, Lanreotide Extended Autogel Duration (LEAD), hormonal control was achieved with lanreotide Autogel given at a dose of 120mg on the extended dosing interval (the first 24 weeks at a 6-week dosing interval with a further 24 weeks at a 6- or 8-week dosing interval) in patients with acromegaly who were well controlled with octreotide LAR 10 or 20mg/4 weeks for ≥6 months.9 Three quarters of the patients (75.8%) maintained IGF-I control 48 weeks after switching to an extended dosing interval. The use of alternative, extensive dosing regimens may improve adherence to treatment and over time can reduce both direct drug costs and indirect costs such as burden on healthcare resources.9 Lanro-Study was an observational, prospective study, that evaluated over 24 months the dosage of lanreotide Autogel 120mg in routine acromegaly care in Poland.7 Patients were eligible for the study eligible if they were treated with lanreotide Autogel 120mg in routine practice for at least three months. Of the 132 patients analyzed, 69 patients (52%) received lanreotide Autogel 120mg/4 weeks and 63 patients (48%) received lanreotide Autogel 120mg at a dosing interval >4 weeks. The Somatuline Depot for Acromegaly (SODA) was an observational study investigating the use of lanreotide Autogel/Depot in clinical practice in the US.11 Patients were eligible for the study if they were treated with lanreotide Autogel/Depot, both patients who were treatment naive and those switched from other agents. Of the 166 patients enrolled in the study, 60mg was the starting dose for 27 (16%) patients, 90mg for 94 (57%) patients, and 120mg for 45 (27%) patients. Almost all patients received injections every 4 weeks, but one patient received 120mg every ≥6 weeks. Unlike the ACROSTART study, not all patients in the Lanro-Study or the SODA study had achieved hormonal control. In the retrospective analysis of the German Acromegaly Register, of patients with acromegaly in Germany, 407 of the 1344 patients (30.3%) had received SSAs.10 Among patients controlled (normalized IGF-I) by lanreotide depot monotherapy (n=27), the median lanreotide dose was 60 (range 50–120) mg every 4 weeks. Overall, the main starting doses reported in the ACROSTART study in Spain are comparable to what has been reported in clinical practice in Poland, the US, and Germany, despite disparity of starting doses and interval dosing.
In the ACROSTART study, both patient satisfaction (93%) as well as adherence (86%) were declared as high by the patient. Although, a limitation regarding the collection of the adherence data was that it was collected from the patients’ clinical history. In the ACROSTART study, 22.8% of the patients managed injections without assistance of healthcare staff, which is higher than has been reported in the real-life study in Poland (2.6%),7 but lower than the real-life study in the US (40.4% at enrollment and 37.9% at 12 months)11; suggesting that in Spain there is a higher opportunity for self-injection. Studies have found an increased preference for self or partner injection over receiving the injection by a healthcare professional.17,18 In the SODA study, injection by healthcare staff was considered less convenient than self or partner injection.11 Furthermore, achievement of biochemical control after 1 year of lanreotide treatment was significantly greater in patients who exclusively were able to home inject (patient or partner only) compared with patients who received injections by healthcare professionals.11 Although it may simply reflect the fact that patients who are better controlled visit the physician's office less often; it is also possible that there was a higher adherence among home injectors. Similar or superior treatment adherence with home injection has been reported in other therapeutic indications that require injections that can be administered by healthcare professionals or home injection.19,20
There are several limitations in this study, including its retrospective and observational design. An important bias to the study was the inclusion only of patients who responded to treatment. Furthermore, the GH and IGF-I values were obtained from several centers, measured as per clinical practice and had not been previously standardized across all centers. As a reflection of real-life practice, the frequency of study visits, biochemical testing, and radiological evaluation were determined by each treating physician. In addition, the distribution of the sample size into subgroups has not permitted to extensively explore specific outcomes according to initial dosing pattern. However, it is speculative to suggest that prolonged interval injections could increase patient convenience by reducing the number of visits and tests. Additionally, pretreatment with other drugs, including SSAs, may have had an impact on the primary outcomes. Nevertheless, the median time to reach hormonal control among patients who were not receiving an SSA when initiating treatment with lanreotide Autogel was similar to the overall population.
In conclusion, real-life treatment with lanreotide Autogel led to an early hormonal control in responder patients, with a high treatment adherence and treatment satisfaction, despite disparity of starting doses and interval dosing and provides clinical evidence that extending the dosing interval to 42 and 56 days in clinical practice is possible
FundingThis study was funded by Ipsen Pharma S.A., Spain.
Conflict of interestCAE received honoraria from IPSEN, Novartis and Pfizer as speaker fees, has served on an advisory board, received lecture fees and sponsorship for travel and accommodation in international scientific meetings from IPSEN, Novartis and Pfizer.
CBC received honoraria from IPSEN, Novartis, Pfizer, AstraZeneca, Lilly, and Novo Nordisk as speaker fees, and received sponsorship for travel and accommodation in international scientific meetings from IPSEN, Novartis and Pfizer.
MMA has received honoraria as speaker fees, has served on advisory board, received lecture fees and sponsorship for travel and accommodation in international scientific meetings from IPSEN, Novartis and Pfizer.
EMT has received honoraria from IPSEN and Novartis as speaker fees.
JAP has served on an advisory board, received lecture fees and sponsorship for travel and accommodation in international scientific meetings from IPSEN, Novartis, and Pfizer.
EMVM has participated in conferences, courses, and steering committees of clinical studies promoted by IPSEN, Novartis, and Pfizer.
MAGR has received sponsorship from IPSEN for attending medical conferences and meetings.
MPdMN has received honoraria as speaker fees and received sponsorship for travel and accommodation in international scientific meetings/trainings from IPSEN, Novartis, and Pfizer.
IHR has participated in conferences and courses organized by Novartis, Pfizer, IPSEN, and has participated in studies sponsored by Novartis, Pfizer, and IPSEN.
GdlCS and AH are IPSEN employees.
EMVM, JAGA, MAGM, ISV, ER, CPF, AMPA do not have conflicts of interest.
The authors thank Francesc Pérez, an Ipsen Pharma employee, for his help during data collection.
Medical writing support was provided by Aurora O’Brate and funded by Ipsen Pharma S.A., Spain.