This randomized crossover clinical trial investigated the effects of substituting legumes for meat consumption in the therapeutic lifestyle change (TLC) diet on leptin and adiponectin concentrations among type 2 diabetic patients.
Material and methodsThirty-one type 2 diabetic patients (24 women, age: 58.1±6.0 years) were randomly assigned to groups designated to consume a legume-free TLC diet or a legume-based TLC diet for 8 weeks. Both diets were similar except for the replacement of two servings of red meat with legumes 3 days per week in the legume-based TLC group. Leptin and adiponectin concentrations were measured at baseline and after the 8-week intervention.
ResultsThe legume-based TLC diet significantly increased adiponectin concentrations in comparison with the legume-free TLC diet. There was no significant change in leptin concentrations after both intervention diets.
ConclusionsLegumes increased serum adiponectin concentrations in type 2 diabetic patients.
Registration number: IRCT201202251640N7.
Este ensayo clínico cruzado aleatorizado investigó los efectos de la sustitución de legumbres por el consumo de carne en la dieta de Cambio Terapéutico en el Estilo de Vida (CTEV) sobre las concentraciones de leptina y adiponectina en pacientes con diabetes tipo 2.
Material y métodosTreinta y un pacientes diabéticos tipo 2 (24 mujeres, edad: 58,1±6,0 años) fueron asignados aleatoriamente a grupos designados para consumir una dieta CTEV libre de legumbres o una dieta CTEV basada en legumbres durante 8 semanas. Ambas dietas fueron similares, excepto por el reemplazo de 2 porciones de carne roja por legumbres 3 días por semana en el grupo de CTEV basado en leguminosas. Las concentraciones de leptina y adiponectina se midieron al inicio y después de la intervención de 8 semanas.
ResultadosLa dieta de CTEV basada en legumbres aumentó significativamente los niveles de adiponectina en comparación con la dieta de CTEV sin legumbres. No hubo cambios significativos en las concentraciones de leptina después de ambas dietas de intervención.
ConclusionesLas leguminosas aumentaron las concentraciones séricas de adiponectina en pacientes diabéticos tipo 2.
Número de registro: IRCT201202251640N7.
Leptin, known as a satiety hormone, an important hormone secreted mainly by adipose tissue, is reported to be a risk factor for type 2 diabetes mellitus by decreasing insulin sensitivity,1 inhibiting insulin secretion,2 and suppressing pro-insulin gene expression.3 Adiponectin, an adipocyte-specific protein with an anti-inflammatory property, has been shown to be negatively associated with insulin resistance and type 2 diabetes.4 Collectively, higher serum concentrations of leptin and/or lower serum concentrations of adiponectin are supposed to be associated with a higher incidence of type 2 diabetes.5–10
Changes in leptin and adiponectin serum concentrations can be modified through approaches such as exercise, drugs, and combination therapy. Moreover, manipulations in dietary components such as substituting monounsaturated fatty acids (MUFA) for carbohydrates,11 consuming a high-fiber diet,12,13 consuming a fish-rich diet,14 consuming more low-glycemic load/index foods,15,16 or decreasing dietary sucrose intake,17 and maintaining a low-calorie diet18 are other useful methods to be considered as applying changes to leptin and adiponectin concentrations.19,20 Legumes are considered excellent sources of soluble and insoluble fibers, vegetable proteins, phytochemicals, vitamins, and minerals.21,22 Interventional and epidemiologic studies have shown an inverse association between legume consumption per se or as part of dietary patterns and leptin16,23,24 and a positive association with adiponectin,15,25 although results have been inconsistent, and associations with diabetic patients are not well investigated.26,27 A thorough search of relevant literature yielded just two studies that have investigated the association between legume consumption and adiponectin concentration among diabetic patients and individuals with family histories of diabetes.25,27
Due to the limited studies available on the association between the substitution of legumes with meat and its effect on leptin and adiponectin concentrations, the current study aimed to determine the effects of substituting legumes with meat consumption on serum leptin and adiponectin concentrations among overweight type 2 diabetic patients.
Materials and methodsParticipantsThe details of the current randomized crossover study, which have also been mentioned in other papers,28 are explained briefly here. Forty-two diabetic patients who attended the clinic of Taleghani hospital, Tehran, Iran, participated in this study. These patients (aged between 50 and 75 years) had a body mass index (BMI) between 25 and 30kg/m2 and did not smoke at all. They were not given insulin therapy and had no signs of cardiac, hepatic, or renal function impairments. They were told to go through a two-week run-in period on a usual diet with no changes in lifestyle; two left the experiment at the end of the run-in period, and the forty remaining patients were randomly (by an assistant, using random sequencing generated in SPSS) chosen to receive one of the two following diets (with the help of an assistant), each for a period of 8 weeks: the legume-free therapeutic lifestyle change (TLC) diet and the legume-based TLC diet. All group allocation was blinded for the investigators. The intervention period was followed by a 4-week washout period, following which the groups underwent the alternate treatment for 8 weeks. Due to poor compliance with the treatment protocol (n=7) and changes in medications (n=2), nine patients dropped out of the study; thus, 31 patients completed the two intervention diets. All participants provided written informed consent. This study was approved by the Research Council and the Ethics Committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (registered in http://www.irct.ir; ID number IRCT201202251640N7).
DietsEach patient was prescribed two intervention diets, namely the legume-free TLC diet and the legume-based TLC diet. The legume-free TLC diet was composed of macronutrients including 50–60% carbohydrates, 15% protein, and 25–35% of energy from fat (<7% saturated fat, up to 20% monounsaturated fat (MUFA), up to 10% polyunsaturated fat (PUFA)) and <200mg cholesterol and 25–30g fiber.29 In fact, these two diets were similar in terms of their ingredients, but 2 servings of red meat were replaced by different types of cooked legumes such as lentils, chickpeas, peas, or beans, 3 days per week in the legume-based TLC diet. It should be noted that half a cup of cooked legumes was considered as one serving of red meat. Patients did not follow a weight-reducing diet, because weight reduction was not supposed to be investigated.
CompliancePrevious studies28,30,31 have thoroughly described the compliance of the participants. Compliance was checked on weekly visits during which the 3-day diet records, including 2 weekdays and 1 weekend day, were collected. Using the NUTRITIONIST III software (version 7.0; N-Squared computing, Salem, OR) designed for Iranian foods, each food and beverage was then analyzed in terms of energy content, macronutrients, and micronutrients. Estimated nutrient intake amounts from all records collected during the intervention were averaged to determine dietary compliance.
Biochemical measurementsFasting venous blood samples were taken after 12–14h overnight fasting at baseline and again after 8 weeks. After consumption of each of the 2 diets, serum was separated and frozen at −70°C on the day of blood collection for biochemical analysis. Intra-assay and inter-assay coefficients of variation of all assays were <5%.
Leptin and adiponectin concentrations were measured using the ELISA method and the ELISA kit (Mercodia Company, Uppsala, Sweden, CAT No. 10-1143-01). Intra-assay variability rates were 8.5% for adiponectin and 1.9% for leptin.
Statistical analysisThe Statistical Package for Social Science (version 15.0; SPSS Inc.) was used for statistical analyses. The independent sample t-test was used to compare age, physical activity, weight, fasting serum glucose, and duration of diabetes; Fisher's exact test was used to analyze gender and medication use between the two intervention groups. Normal distributions of leptin and adiponectin were checked with the Kolmogorov-Smirnov test, and because distributions of biomarkers were not normal, the logarithmically transformed values of these variables were used in all analyses and their geometric means have been reported. To calculate the changes in leptin and adiponectin, the baseline and end-of-intervention values were used; a comparison was made between baseline and end-of-intervention values in each group using the paired t-test. Results are expressed as mean±SE, and statistical significance was defined at p<0.05.
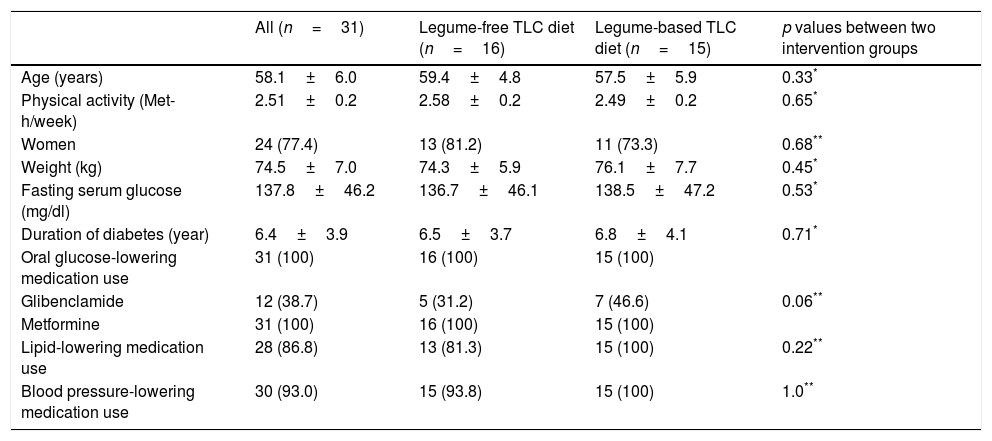
ResultsThirty-one diabetic patients (77.4% women) with a mean age of 58.1±6.0 years and BMI of 27.8±1.3kg/m2 completed the current crossover study. Baseline characteristics for participants before initial intervention in each of the intervention groups are shown in Table 1. There were no significant differences in the distribution of patients with respect to age, gender, weight, physical activity levels, fasting serum glucose, duration of diabetes or medication use between the legume-free TLC diet and the legume-based TLC diet groups. The participants were similar in terms of their activity levels across all study periods (legume-free TLC diet group: 2.58±0.2; legume-based TLC diet: 2.49±0.2). Dosages of antihypertensive, lipid-lowering drugs, and oral glucose-lowering medications remained unchanged during the intervention period.
Baseline characteristics of participants at initiation of intervention diet.
| All (n=31) | Legume-free TLC diet (n=16) | Legume-based TLC diet (n=15) | p values between two intervention groups | |
|---|---|---|---|---|
| Age (years) | 58.1±6.0 | 59.4±4.8 | 57.5±5.9 | 0.33* |
| Physical activity (Met-h/week) | 2.51±0.2 | 2.58±0.2 | 2.49±0.2 | 0.65* |
| Women | 24 (77.4) | 13 (81.2) | 11 (73.3) | 0.68** |
| Weight (kg) | 74.5±7.0 | 74.3±5.9 | 76.1±7.7 | 0.45* |
| Fasting serum glucose (mg/dl) | 137.8±46.2 | 136.7±46.1 | 138.5±47.2 | 0.53* |
| Duration of diabetes (year) | 6.4±3.9 | 6.5±3.7 | 6.8±4.1 | 0.71* |
| Oral glucose-lowering medication use | 31 (100) | 16 (100) | 15 (100) | |
| Glibenclamide | 12 (38.7) | 5 (31.2) | 7 (46.6) | 0.06** |
| Metformine | 31 (100) | 16 (100) | 15 (100) | |
| Lipid-lowering medication use | 28 (86.8) | 13 (81.3) | 15 (100) | 0.22** |
| Blood pressure-lowering medication use | 30 (93.0) | 15 (93.8) | 15 (100) | 1.0** |
Met-h, metabolic equivalent hours; TLC, therapeutic lifestyle change.
Data is in mean±SD or n (%).
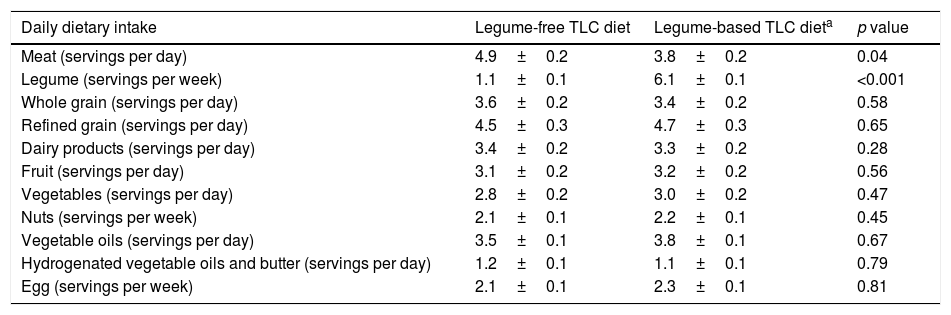
Analysis of the 3-day diet self-record showed that, compared with the legume-based TLC diet, dietary intake of meat was significantly higher, and intake of legumes was significantly lower in the legume-free TLC diet.28 No significant difference was shown in other dietary food groups (Table 2).
Dietary intakes of the participants by intervention diet.
| Daily dietary intake | Legume-free TLC diet | Legume-based TLC dieta | p value |
|---|---|---|---|
| Meat (servings per day) | 4.9±0.2 | 3.8±0.2 | 0.04 |
| Legume (servings per week) | 1.1±0.1 | 6.1±0.1 | <0.001 |
| Whole grain (servings per day) | 3.6±0.2 | 3.4±0.2 | 0.58 |
| Refined grain (servings per day) | 4.5±0.3 | 4.7±0.3 | 0.65 |
| Dairy products (servings per day) | 3.4±0.2 | 3.3±0.2 | 0.28 |
| Fruit (servings per day) | 3.1±0.2 | 3.2±0.2 | 0.56 |
| Vegetables (servings per day) | 2.8±0.2 | 3.0±0.2 | 0.47 |
| Nuts (servings per week) | 2.1±0.1 | 2.2±0.1 | 0.45 |
| Vegetable oils (servings per day) | 3.5±0.1 | 3.8±0.1 | 0.67 |
| Hydrogenated vegetable oils and butter (servings per day) | 1.2±0.1 | 1.1±0.1 | 0.79 |
| Egg (servings per week) | 2.1±0.1 | 2.3±0.1 | 0.81 |
TLC, therapeutic lifestyle change.
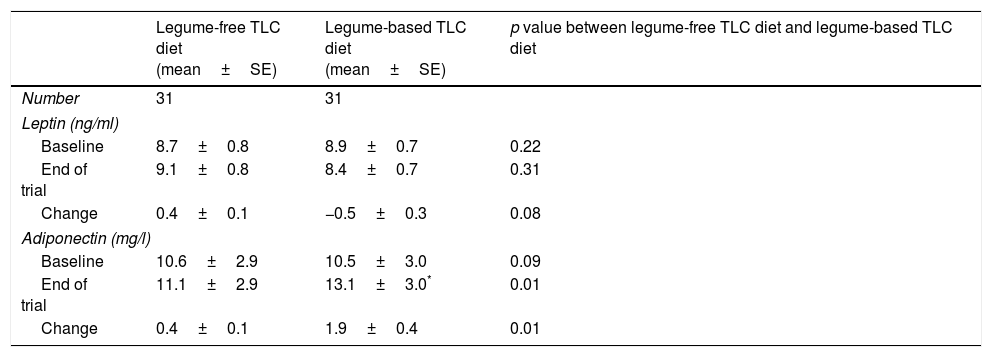
The geometric means of leptin and adiponectin at baseline and after 8 weeks of intervention in type 2 diabetic patients are shown in Table 3. After consumption of the legume-based TLC diet, adiponectin increased significantly from baseline values. Compared with the legume-free TLC diet, the legume-based TLC diet significantly increased adiponectin (change: 1.9±0.4, p<0.01). Leptin concentrations did not change significantly after consumption of either the legume-free TLC diet or the legume-based TLC diet. The legume-based TLC diet was the same as the legume-free TLC diet, except that 2 servings of red meat were replaced with different cooked legumes such as lentils, chickpeas, peas, and beans 3 days per week.
Geometric means of leptin and adiponectin at baseline and after 8 weeks of intervention in type 2 diabetic patients.
| Legume-free TLC diet (mean±SE) | Legume-based TLC diet (mean±SE) | p value between legume-free TLC diet and legume-based TLC diet | |
|---|---|---|---|
| Number | 31 | 31 | |
| Leptin (ng/ml) | |||
| Baseline | 8.7±0.8 | 8.9±0.7 | 0.22 |
| End of trial | 9.1±0.8 | 8.4±0.7 | 0.31 |
| Change | 0.4±0.1 | −0.5±0.3 | 0.08 |
| Adiponectin (mg/l) | |||
| Baseline | 10.6±2.9 | 10.5±3.0 | 0.09 |
| End of trial | 11.1±2.9 | 13.1±3.0* | 0.01 |
| Change | 0.4±0.1 | 1.9±0.4 | 0.01 |
Data is in mean±SE.
The results of this randomized crossover study show that replacing meat with legumes in a TLC diet caused increased levels of serum adiponectin concentration among type 2 diabetic patients which had nothing to do with weight change. Leptin concentration was not affected by either the TLC diet or the TLC diet with legumes.
The number of studies investigating the relationship between legume consumption and adiponectin concentration in humans is limited.15,25,27 In the current study, inclusion of legumes in a TLC diet increased adiponectin concentrations among overweight diabetic patients. Consistent with the current findings, a crossover feeding trial in healthy overweight and obese adults showed that a low-glycemic load diet (containing legumes) increased adiponectin concentration after 28 days.15 In addition, a prospective study among diabetic women from the Nurses’ Health Study showed that median plasma adiponectin concentrations were 23% higher among women following a Mediterranean-type diet; however, no significant association was found between adiponectin concentration and servings per day of legumes.25 On the other hand, in a 6-week randomized crossover study on subjects with a family history of diabetes, consumption of 4 servings per week of legumes was not significantly associated with adiponectin concentration.27 Overall, findings from previous studies have shown that legume consumption has beneficial effects on adiponectin concentrations among participants with diabetes, overweight, and obesity,15,25 but not among healthy participants without hyperglycemia or insulin resistance.27 Legume consumption improves insulin resistance, inflammatory markers, and oxidative stress among diabetic patients, effects which may be mediated through adiponectin concentration.30,32,33 Improvements in inflammation biomarkers and oxidative stress could explain the increased adiponectin among diabetic patients. Adiponectin is an antidiabetic, anti-inflammatory factor and reduces oxidative stress.34,35 In addition, adiponectin improves insulin resistance, hyperglycemia, and hyperlipidemia.36
Leptin concentration has been reported to increase among diabetic patients, possibly due to its association with inflammation level.37–39 The present study showed no significant effect of legume consumption on leptin concentration in a TLC diet. The results of the current study are in accordance with a prospective study from the National Health and Nutrition Examination Survey III, in which leptin was not independently associated with dietary intake or dietary pattern.26 However, western dietary patterns increased leptin concentrations in US male health professionals23 and in Chinese men and women,24 which may attributed to the fact that the western dietary pattern is an unhealthy pattern causing weight gain and obesity, a status in which leptin concentrations increase significantly. In contrast, in a 4-week crossover feeding trial of non-smoking men at high risk for colorectal cancer, consumption of 1.5 cups of cooked bean mixture per 2000kcal reduced fasting leptin concentrations.16 Diabetic patients usually show a higher serum leptin concentration.2 In the present study, no change in participant weight was seen; thus, weight stability in this study could be a reason for the lack of an effect of legume consumption on leptin concentration. One of the main mechanisms for decreasing leptin in diabetic patients is consumption of a low-caloric diet and the subsequent weight loss.40
In the current study, exclusion of participants with receiving insulin therapy and high control in compliance of diet interventions limited the generalizability of results to a community setting. In addition, the aim of the study was to investigate the effects of substituting legumes for meat consumption in an isoenergetic TLC diet without energy restriction. Therefore since the patients did not follow a weight-reducing diet, we recruited only overweight diabetic patients. This limited the generalizability of results to obese diabetic patients.
The current study had some limitations. First, specific dietary recommendations were considered for the intervention diets and pre-prepared diets were not given to the participants; thus, the participants may not have followed the diets as carefully as they did in trials where prepared food was provided. In addition, due to financial restrictions, the biochemical index of legume consumption was not measured due to financial restrictions; however, the findings indicate that following the legume-based TLC diet has beneficial effects on adiponectin concentrations. Another limitation of the present study is that the trial (dietary intervention) was non-blinding by nature. The rate of non-compliance was also a limitation, because about one-quarter of diabetic patients were eliminated in the follow-up. It should be noted that this crossover trial was carried out over a long period; thus, it is only natural that it would have a greater number of patient exclusions.
ConclusionIn conclusion, the current randomized crossover study among adults from Tehran showed that the inclusion of legumes in a TLC diet increases the adiponectin concentration; however, no significant association was observed regarding leptin concentration.
Funding sourcesThis work was supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran [grant 767].
Authors’ contributionsS.H.N. conceived and designed the study. S.H.N. and S.H. performed statistical analysis and interpretation of data and wrote the manuscript. P.M. and F.A. supervised the conduct of the study and revised the manuscript. All authors have approved the final article.
Conflict of interestNone declared.
The authors would like to express their appreciation to the participants who participated in this study. They also wish to acknowledge Ms. Niloofar Shiva for critical editing of English grammar and syntax of this manuscript.