Nivolumab is an anti-cancer monoclonal antibody that inhibits PD1 and modulates T-cell response. It has been shown to significantly improve survival in several types of cancer, but clinical trials have also reported an increased risk of developing immune-related adverse events (IRAEs). Endocrine IRAEs may be particularly relevant.
ObjectiveTo comprehensively evaluate the clinical presentation of endocrine IRAEs in patients with lung cancer treated with nivolumab. Potential risk factors are analyzed, and strategies for IRAE management are proposed.
MethodsForty consecutive patients treated with nivolumab for advanced non-small cell lung cancer (NSCLC) were studied, paying particular attention to development of endocrine IRAEs (thyroid, hypophyseal, adrenal, or pancreatic) and clinical outcome.
ResultsThyroid function changes were found in 9 patients (22.5%), of which six developed hypothyroidism and three had hyperthyroidism after a median of 3.8 and 2.3 cycles of nivolumab respectively. Only one patient had thyroid-related symptoms. Thyroid autoimmunity was negative in all cases. Hyperthyroid patients showed no uptake in iodine scintigraphy, and their hormone values returned to normal in less than six months. Nivolumab was discontinued for toxicity in one patient. One patient with hyperthyroidism also developed autoimmune diabetes, and one patient with hypothyroidism also had hypogonadism. After a median follow-up of 7.6 months, 25 patients (62.5%) showed response to nivolumab. Univariate and multivariate analyses showed no differences between patients who developed thyroid changes and those who did not.
ConclusionsThyroid changes after treatment with nivolumab are common and warrant active laboratory monitoring. The underlying mechanisms and their relevance deserve further research.
Nivolumab es un anticuerpo monoclonal que ejerce acción anti-tumoral mediante la inhibición de PD1 y modulación de la respuesta de las células T. Ha demostrado un aumento significativo en la supervivencia de distintos tipos de cáncer, pero también se ha reportado un incremento en el riesgo de desarrollar eventos adversos relacionados con la inmunidad (IRAEs). Los IRAEs endocrinos son particularmente relevantes.
ObjetivosEvaluar de forma detallada la presentación clínica de los IRAEs endocrinos en pacientes con cáncer de pulmón refractario tratados con nivolumab. Se analizan potenciales factores de riesgo y se proponen estrategias para su manejo.
Material y métodosSe estudiaron 40 pacientes consecutivos con cáncer de pulmón de células no pequeñas (NSCLC) tratados con nivolumab. Se realizó el seguimiento prestando especial atención al desarrollo de los IRAEs endocrinos (tiroides, hipófisis, adrenal o páncreas) y su evolución clínica.
ResultadosSe detectaron alteraciones de la función tiroidea en 9 casos (22,5%): 6 hipotiroidismo y 3 hipertiroidismo, tras una mediana de 3,8 y 2,3 ciclos de nivolumab, respectivamente. Solo un paciente desarrolló síntomas relacionados. La autoinmunidad tiroidea fue negativa en todos los casos. La gammagrafía fue negativa en todos los casos de hipertiroidismo y los valores hormonales volvieron a la normalidad en menos de 6 meses. Se suspendió nivolumab en un caso debido a toxicidad. Uno de los pacientes con hipertiroidismo también desarrolló diabetes autoinmune, y uno de los pacientes con hipotiroidismo también presentaba hipogonadismo. Tras una mediana de seguimiento de 7,6 meses, 25 pacientes (62,5%) presentaron respuesta favorable al nivolumab. El análisis uni y multivariante no mostró diferencias entre los pacientes que desarrollaron alteraciones tiroideas y los que no.
ConclusionesLas alteraciones tiroideas tras el tratamiento con nivolumab son frecuentes y requieren una vigilancia activa. Los mecanismos subyacentes y su relevancia aún no se conocen en profundidad.
With recent advances in the knowledge and understanding of immunology and cancer biology, there has been an increase in the use of immune checkpoint inhibitors (ICPI) for the treatment of malignancies. Specifically, these therapies are commonly based on mechanisms of boosting the negative immunoregulatory receptors on T cell surface to enhance the host immunity against tumor cells. In this regard, nivolumab is a monoclonal antibody that binds and blocks the activation of the programmed cell-death-1 (PD1) receptor, which is expressed on T-cells and binds to its ligands PD-L1 and PD-L2 on cancer cells and other immune and non-immune cells. Thus, it increases the anti-tumor T-cell response by blocking the interaction of PD1 and PD-L1 to prevent T-cell inactivation at the tumor site. In fact, a significant improvement in survival outcomes has been demonstrated with nivolumab in several types of solid tumors, including melanoma and lung cancer, in comparison to chemotherapy.1–5
As a counterpart to this antineoplastic activity, ICPI may contribute to the development of a unique set of mechanism-based toxicities, termed immune-related adverse events (IRAEs), as a consequence of impaired self-tolerance from loss of T-cell inhibition.6 Endocrine IRAEs, although less acknowledged at the beginning, are emerging as a topic of increasing relevance and interest, especially given their potential highly symptomatic nature, the availability of treatments to ameliorate them if they occur, and the interest in discovering the underlying mechanisms involved.4,6–9
Most of the information available on these potential side effects derives from preclinical and laboratory-funded randomized studies,10,11 and only recently there have been publications regarding post-commercial and observational clinical real world data in case reports and case-series.12–13
The aim of this study is to present a comprehensive evaluation of the clinical presentation of endocrine IRAEs in a large cohort of patients with refractory lung cancer from a single center treated with nivolumab. We use clearly defined criteria to establish the occurrence of endocrine IRAEs, we provide detailed longitudinal follow-up, and we suggest potential risk factors for the development of IRAE, specifically regarding the thyroid, and devise strategies for their approach and clinical management, including the consideration of continuing to receive these ICPI.
Subjects and methodsStudy populationWe retrospectively studied 40 consecutive patients with previously treated non-small-cell lung cancer (NSCLC) in our center who were treated with nivolumab from February 2016 to April 2017. We followed them up regarding their clinical outcome, and also regarding the development of endocrine IRAEs entailing thyroid, pituitary, adrenal or pancreatic dysfunction. The study was approved by the Ethics Committee of the Hospital La Princesa, it was in compliance with the Helsinki Declaration, and all patients signed a written informed consent prior to beginning nivolumab.
Evaluation of patients’ clinical and biochemical dataData regarding medical history, physical examination, laboratory and radiological work-up were obtained from clinical records. The following baseline evaluations were collected at the time of initiating nivolumab: sex, age, type and stage of lung cancer, number of treatments received before starting nivolumab, co-existing illnesses and comorbidities, including diabetes mellitus and thyroid dysfunction, smoking habit history, family history of autoimmune conditions and Eastern Cooperative Oncology Group (ECOG) performance status. Laboratory assessment specifically included blood count, thyroid function tests, fasting blood glucose and HbA1c levels. Additional hormonal evaluations were performed in accordance to clinical signs and symptoms. Follow-up was performed as currently recommended14,15 with periodic monitoring of physical examination and clinical and biochemical work-up.
Treatment with nivolumab and follow-up evaluationNivolumab was administered as an intravenous infusion of 3mg/kg every 14 days, and continued until disease progression or unacceptable toxicity. Screening thyroid function tests (TFTs), consisting of thyroid-stimulating hormone (TSH) and free-thyroxine (FT4) were completed in all patients at baseline and monthly during subsequent follow-up visits. Blood tests for cortisol, adrenocorticotropin (ACTH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, and either estradiol or testosterone were performed according to clinical indications and/or investigator preference. Standard quantitative enzymatic or radioimmunometric assays were performed according to the manufacturers’ instructions for the indicated hormone tests. Whenever an endocrine IRAE was detected, specific autoimmunity was determined (anti-thyroglobulin antibodies, TGAb, anti-thyroperoxidase antibodies, TPOAb, thyroid-stimulating hormone receptor antibodies, TSHRAb, anti-insulin, IAA-Ab, anti-glutamic acid decarboxylase, anti-GAD-Ab).
Follow-up evaluation included tumor response according to the usual Response Evaluation Criteria for Solid Tumors (RECIST), and patients were grouped into two categories: partial response (reduction of at least 30% of the target lesions), complete response (complete disappearance of lesions) and stable disease (when neither response nor tumor progression criteria are met) on one hand, versus disease progression (increase of at least 20% of the target lesions, with an increase of at least 5mm) on the other. Number of nivolumab infusions until the last follow-up were recorded in all cases, and date and reasons were documented in cases in which nivolumab was withdrawn.
Definition of endocrine immune-related adverse eventsImmune-related primary hypothyroidism was defined by the presence of a TSH level ≥5mIU/l alone with or without a low FT4. Immune-related thyroiditis in the hyperthyroid phase was defined by the presence of a suppressed TSH level with an elevated FT4. Graves’ hyperthyroidism was defined as biochemical hyperthyroidism and positive TSHRAb, following recent guidelines.16 Other thyroid dysfunction, excluding that related to hypophysitis, hypothyroidism, and thyroiditis, was defined by the presence of a low or elevated TSH level with a low or normal FT4, as suggested previously.17
Following previous recommendations,18 hypophysitis was identified by the presence of: (i) secondary adrenal insufficiency, defined by the presence of onset of symptoms of adrenal insufficiency associated with biochemically proven low or suppressed serum cortisol levels with inappropriately low ACTH levels in the absence of exogenous steroid treatment; dynamic ACTH stimulation testing was not performed; and (ii) suspected or suspicious for secondary hypothyroidism, defined by a low FT4 level with either a normal or suppressed TSH level that did not normalize on subsequent testing. If pituitary biochemical abnormalities were detected, magnetic resonance imaging was performed. Cases considered as suspicious for central hypothyroidism were differentially diagnosed from euthyroid sick syndrome based on the laboratory tests consistent with central hypothyroidism with co-existing central adrenal insufficiency and/or MRI evidence of hypophysitis. A few patients were screened for secondary hypogonadism when other pituitary deficiencies were thought to be developing. We did not evaluate growth hormone or insulin-like growth factor levels, and prolactin levels were also only determined if hypophysitis was suspected.
Acute development of autoimmune diabetes was diagnosed based on plasma glucose criteria, either the fasting plasma glucose or the 2-h plasma glucose (2-h PG) value after a 75-g oral glucose tolerance test (OGTT), rather than on A1C criteria, together with positivity to one or more diabetes-related autoimmune markers (islet cell autoantibodies and autoantibodies to GAD (GAD65), insulin, and the tyrosine phosphatases IAA), according to recent guidelines.18
Statistical analysisDescriptive results were expressed as mean±standard deviation and median (interquartile range) for normally- and not-normally-distributed continuous variables, respectively. Categorical variables were summarized as frequencies and percentages. Comparison between categorical groups was assessed with Chi square test (Fisher's exact test as required), whilst continuous variables were compared by U-Mann–Whitney, Kruskal–Wallis, t-test for independent samples or t-test for related samples, accordingly. Patients’ baseline features, and those related with nivolumab treatment, were examined for their influence on the development of thyroid dysfunction using univariate binary logistic regression analysis. For those variables identified as potential predictors, multivariate logistic regression analysis was performed. Cox and Kaplan–Meyer regression curves were performed for survival analysis. The p-values were two-sided and statistical significance was considered when p<0.05. All statistics were performed using SPSS version 22.0 (IBM SPSS Statistics Inc., Chicago, IL, USA).
ResultsWe collected data from 40 patients (13, 32.5%, female), aged 69 (38–86) years old, with a median ECOG performance status 1 (0–2) (2 patients with ECOG=0, 20 with ECOG=1 and 18 with ECOG=2), and with an active smoking habit in 32 cases (80%). NSCLC was classified as non-squamous cells in 17 cases (42.5%), squamous cells in 22 (52.5%), and 2 cases were classified as none other specified (NOS) NSCLC. Fifty percent of patients received nivolumab as a second-line treatment, whilst the other half had already received two or more previous chemotherapy regimens before undergoing nivolumab treatment (mean number of previous chemotherapy regimens before nivolumab 1.8, range 1–5). Thirty-one patients (77.5%) presented metastatic disease, and the rest had unresectable locally advanced disease. All patients had normal thyroid function tests in the absence of hormonal replacement treatment before the first dose of nivolumab was administered, although there were two exceptions: two patients with amiodarone-related hypothyroidism were adequately controlled with levothyroxine treatment.
After a follow-up of 7.6±3.9 (1–15) months, 11 patients (27.5%) exhibited stable disease, 13 (32.5%) partial response and 1 patient (2.5%) had a complete response, whilst 15 patients (37.5%) showed disease progression. Nivolumab was withdrawn in 19 patients (47.5%); reasons for withdrawal were tumor progression in 11 cases, unacceptable severe toxicities in 2 (one patient developed severe hyperthyroidism – see below and Table 1 – and the other one developed immune-related colitis), and death in 6 patients (3 of them for cancer-related causes).
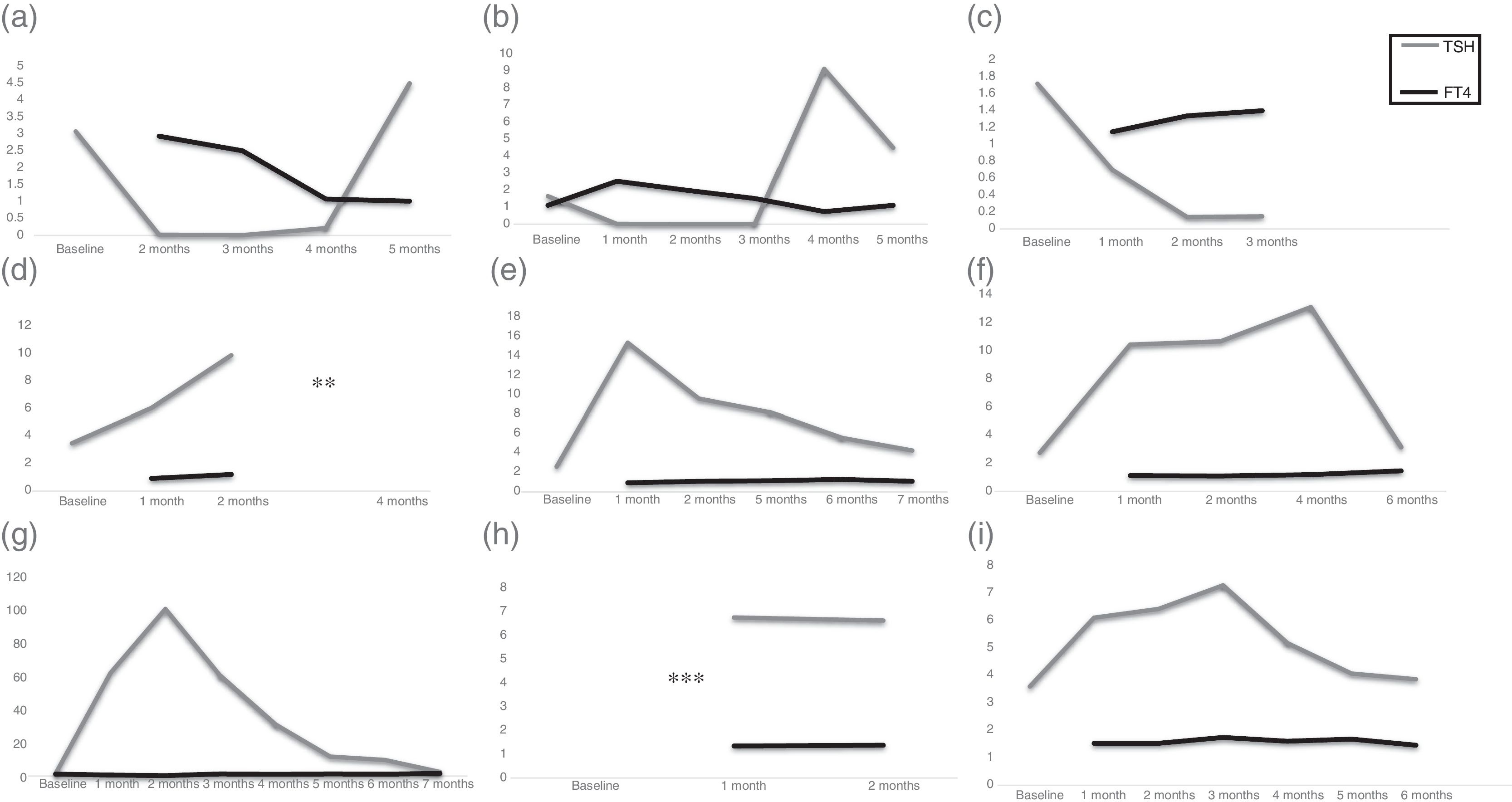
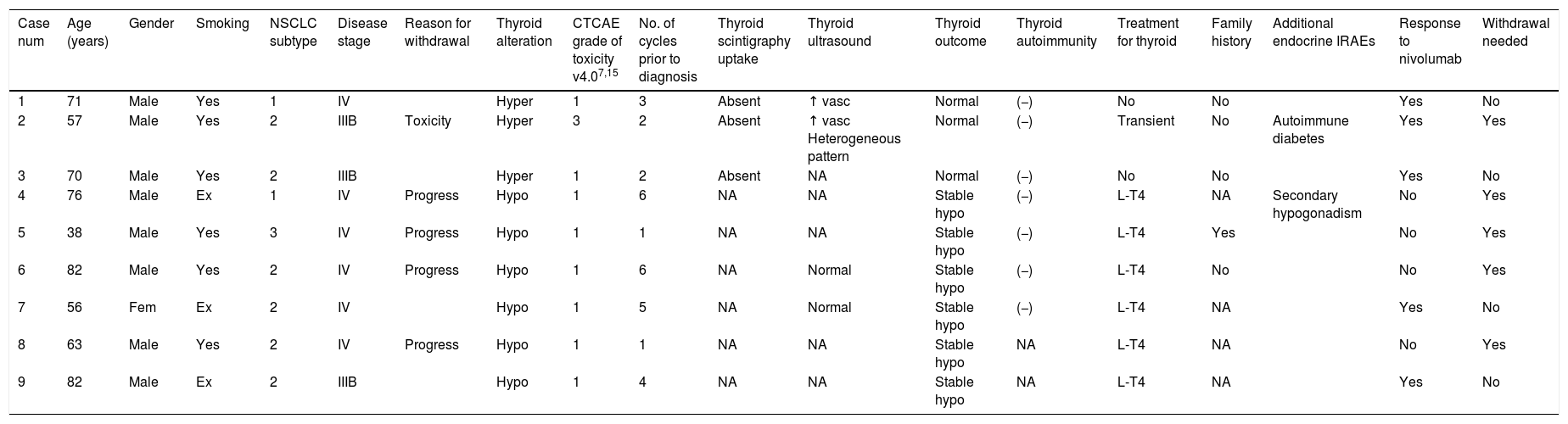
Complete description of the 9 patients who suffered thyroid function test abnormalities after nivolumab treatment.
| Case num | Age (years) | Gender | Smoking | NSCLC subtype | Disease stage | Reason for withdrawal | Thyroid alteration | CTCAE grade of toxicity v4.07,15 | No. of cycles prior to diagnosis | Thyroid scintigraphy uptake | Thyroid ultrasound | Thyroid outcome | Thyroid autoimmunity | Treatment for thyroid | Family history | Additional endocrine IRAEs | Response to nivolumab | Withdrawal needed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Male | Yes | 1 | IV | Hyper | 1 | 3 | Absent | ↑ vasc | Normal | (−) | No | No | Yes | No | ||
| 2 | 57 | Male | Yes | 2 | IIIB | Toxicity | Hyper | 3 | 2 | Absent | ↑ vasc Heterogeneous pattern | Normal | (−) | Transient | No | Autoimmune diabetes | Yes | Yes |
| 3 | 70 | Male | Yes | 2 | IIIB | Hyper | 1 | 2 | Absent | NA | Normal | (−) | No | No | Yes | No | ||
| 4 | 76 | Male | Ex | 1 | IV | Progress | Hypo | 1 | 6 | NA | NA | Stable hypo | (−) | L-T4 | NA | Secondary hypogonadism | No | Yes |
| 5 | 38 | Male | Yes | 3 | IV | Progress | Hypo | 1 | 1 | NA | NA | Stable hypo | (−) | L-T4 | Yes | No | Yes | |
| 6 | 82 | Male | Yes | 2 | IV | Progress | Hypo | 1 | 6 | NA | Normal | Stable hypo | (−) | L-T4 | No | No | Yes | |
| 7 | 56 | Fem | Ex | 2 | IV | Hypo | 1 | 5 | NA | Normal | Stable hypo | (−) | L-T4 | NA | Yes | No | ||
| 8 | 63 | Male | Yes | 2 | IV | Progress | Hypo | 1 | 1 | NA | NA | Stable hypo | NA | L-T4 | NA | No | Yes | |
| 9 | 82 | Male | Ex | 2 | IIIB | Hypo | 1 | 4 | NA | NA | Stable hypo | NA | L-T4 | NA | Yes | No |
NSCLC, non-small-cell lung cancer; subtypes 1: non-squamous, 2: squamous, 3: none other specified; “progress”: progression; “hyper”: hyperthyroidism; “hypo”: hypothyroidism; number of cycles prior to diagnosis refers to the number of cycles of nivolumab received before developing the thyroid function abnormality; “↑”: increase; “NA”: not available”; “vasc”: vascularization; “L-T4”: levothyroxine replacement therapy; “(−)”: negative; “(+)”: positive; “IRAEs”: immune-related adverse events.
Complete description of patients who suffered thyroid function test abnormalities after nivolumab treatment.
We detected abnormal thyroid function test results in 9 patients (22.5%). Specifically, 4 patients developed hypothyroidism and the 2 patients with a prior history of stable hypothyroidism exhibited an increase in their TSH values after 3.8 (1–6) doses of nivolumab; and 3 patients developed hyperthyroidism after 2.3 (2–3) doses. Table 1 summarizes clinical data and Fig. 1(a)–(i) shows thyroid hormone test values for the 9 patients who developed nivolumab-induced thyroid dysfunction. It is worth emphasizing that only one of the patients who developed hyperthyroidism (case number 2 in Table 1, Fig. 1b) exhibited related symptoms, consisting of palpitations, tachycardia and anxiety, which was categorized as severe hyperthyroidism (grade 3 according to the Common Terminology Criteria for Adverse Events, CTCAE, version 4.0) of National Institutes of Health, National Cancer Institute)9,19 and required transient withdrawal of nivolumab and symptomatic treatment. The rest of the patients did not develop new symptoms, and their ECOG performance status did not change at the time of detecting abnormalities in thyroid function tests. Family history of thyroid dysfunction was not informative: 4 of the 5 patients for whom this information was available did not have a previous family history which could predict the possibility of developing thyroid alterations.
Schematic representations of TSH and FT4 values for one of the 9 patients with nivolumab-induced thyroid dysfunction. Figs. (a)–(i) correspond to cases 1–9, respectively, from Table 1. Values are shown in μU/ml for TSH (reference range 0.5–5μU/ml) and ng/dl for FT4 (reference values 0.7–1.8ng/dl). Baseline hormonal values refer to those prior to the first dose of nivolumab. Horizontal axis shows the time lapse between hormonal determinations. ** For case number 4 (d), TSH/FT4 values were not available after 3 months, but clinical record recalls “within normal range after L-T4 adjustment”. *** For case number 8 (h) clinical records acknowledged normal TSH/FT4 values prior to the start of nivolumab and 3 months after, with L-T4 adjustment.
Interestingly, the 3 patients who developed hyperthyroidism had negative thyroid autoimmunity and absence of uptake in iodine scintigraphy. Hormonal values returned to normal in less than 6 months in 2 patients. In the third patient, although recovery could be foreseen due to stability of TSH and FT4 values, full recovery could not be evidenced because he died for non-cancer-related causes. In contrast, patients who developed hypothyroidism, and those with a previous history of hypothyroidism who underwent sudden TSH elevations persisted in their need of increased levothyroxine dose. Morphological or autoimmune findings were not informative, and there was no relationship between prior history of imaging (such as baseline computed tomography with intravenous contrast) and the development of thyroid alterations.
We did not find differences in baseline characteristics (age, sex, type of lung cancer, disease stage, ECOG performance status, smoking history, or number of lymphocytes) between patients who developed thyroid alterations and those who did not. Similarly, we could not evidence significant differences regarding patients’ best response to treatment, i.e., patients who developed thyroid alterations did not exhibit a better or worse tumor response to nivolumab than those who did not. In this regard, univariate or multivariate analysis of the available clinical and analytical data could not demonstrate any potential risk factors involved in the development of thyroid abnormalities in our cohort of patients.
The only patient who exhibited hyperthyroid symptoms after nivolumab also developed autoimmune diabetes. This patient had a previous 2-year history of type 2 diabetes, which was adequately controlled with lifestyle modifications and 1700mg/day of metformin. However, at the time in which hyperthyroidism was observed, biochemical laboratory work-up evidenced concomitant diabetic ketoacidosis, which required high doses of intravenous insulin to achieve optimal control. Subsequent evaluation denoted positivity of pancreatic autoantibodies (IA2, IAA and antiGAD), and subcutaneous insulin was adjusted. This finding prompted an active search of other potential endocrine IRAEs (specifically, pituitary and adrenal), but no additional hormonal alteration was evidenced. Nivolumab was temporarily withdrawn due to the toxicities observed, but was then reintroduced, with subsequent partial response and no new adverse events.
In the active follow-up of the cohort of patients, we did not identify any clinical sign or symptom of any further endocrine alteration, except for secondary hypogonadism in one patient. This patient was further evaluated with a complete hormonal profile due to progressive asthenia, muscle weakness and loss of libido. Results showed low total testosterone and FSH levels, but no biochemical abnormalities suggesting adrenal or other pituitary dysfunction, and an unremarkable pituitary magnetic resonance imaging (MRI). After discussing treatment options, the patient did not want to receive testosterone replacement, and wanted to rather focus on his lung cancer recovery. No steroids were administered.
DiscussionIn this study, we comprehensively describe the outcome of thyroid function in lung cancer patients who received treatment with the ICPI nivolumab in a single experienced center. The prevalence of this alteration in our cohort (22.5%) could be considered higher than that reported in the literature (1–16%).9,12,13,14,20 However, not all studies have evaluated this adverse event routinely, and some of them report IRAEs in general. In fact, the unexpressive clinical scenario regarding thyroid symptoms in these patients, the potential overlap with cancer-related constitutional symptoms, and the little therapeutic intervention performed may disguise a higher prevalence than what has been previously reported. For instance, in a phase II trial in Japanese patients with advanced melanoma reported by Deeks et al.,21 45.7% of patients developed IRAEs, with the most common adverse event being hypothyroidism, and in another recent study, thyroid dysfunction occurred in 3 out of the 14 patients who underwent nivolumab treatment.22 In line with these reports, a prevalence like the one observed in our study could be considered more realistic in the clinical setting.
In a recent case-series of five patients,23 nivolumab-induced thyrotoxicosis seemed to be associated with painless thyroiditis, but no cases of Graves’ disease or Hashimoto's thyroiditis. They suggested that nivolumab reduces immune tolerance, leading to transient thyroiditis. In another study in 10 patients who developed thyroid dysfunction after nivolumab,24 6 patients exhibited the classical features of painless thyroiditis, all with negative autoimmunity, whilst 4 were directly diagnosed with hypothyroidism without a prior hyperthyroid phase, and these patients did have positivity for anti-thyroglobulin and anti-thyroperoxidase antibodies. And in another recent cohort of patients with metastatic melanoma treated with either of the two anti-PD1 monoclonal antibodies, 21 out of 24 patients were initially diagnosed with some form of hyperthyroidism, which then evolved variably, and the remaining 3 were directly diagnosed with hypothyroidism, and only 7 patients had positive thyroid autoimmunity.20
In our cohort, of the 9 patients who presented with thyroid alterations, the 3 who developed a thyrotoxicosis-like clinical picture (biochemical primary hyperthyroidism, absent thyroid scintigraphy uptake and negative thyroid autoantibodies), returned to normal thyroid hormone levels in less than 6 months, whilst the 6 patients who developed increased TSH values remained as such, and required either initiation of levothyroxine replacement therapy for those with newly diagnosed hypothyroidism, or adjustment in the 2 cases who had a prior history of amiodarone-induced thyroid dysfunction. In contrast to the report by Orlov et al.24 but in a similar way to the study by Sznol et al.,20 thyroid autoimmunity was negative in 7 of the 9 patients in our series, regardless of the initial form of onset of thyroid dysfunction. It could be speculated that hypothyroid patients had previously undergone a thyrotoxic phase, and were now in a recovery phase. However, the frequent hormonal evaluation performed did not identify this prior treatment-induced hyperthyroid state, and none of the patients with thyroiditis became hypothyroid. This favors the hypothesis of two distinct groups of thyroid alterations following nivolumab: a transient painless thyroiditis and a direct hypothyroid effect, despite the negative autoimmunity in all cases and no relevant morphological issues. Although central hypothyroidism is sometimes difficult to differentiate from euthyroid sick syndrome, we did not encounter such a clinical scenario in our cohort, since all patients who developed hypothyroidism in our series exhibited primary hypothyroidism.
Our findings could provide new insights to the potential mechanisms involved in the development of endocrine adverse events in the setting of treatment with ICPI. So far, we know that PD1/PDL1 engagement inhibits both T-cell proliferation and the production of proinflammatory Th1 cytokines, including IFN-g and IL-2.25 Blockade of this axis seems to, at least partly, release effector T cell responses against tumor-associated self neo-antigens, resulting in autoimmunity through direct self-antigen-mediated cytotoxicity, the development T cell-dependent self-antibodies and through subsequent bystander damage following tissue inflammation.26 Then, tissue damage triggers the autoimmune loop, with a further release of tissue self-antigens for priming of more self-reactive T cells. But the reason for the specific target toward the pituitary, thyroid and adrenal glands remains unclear.14,27 Given the clinical picture of thyroid abnormalities with negative autoimmunity, it could be presumed that the mechanisms leading to the development of thyroid alterations involve more than just the autoimmune enhancement secondary to nivolumab. In this regard, hypothetically, cellular mechanisms involving the Th1/Th17 pathway and apoptosis, and not only Th2 and humoral pathways, could play a relevant role.28 In fact, although some reports suggest the development of anti-thyroglobulin, anti-thyroid peroxidase, or anti-TSH receptor antibodies as the underlying mechanisms,29,30 the arousal of antibodies has not been reported in all cases.17
It would be interesting to investigate this issue in the future, and evaluate thyroid autoantibodies or prior family history for patients who are potential candidates to these treatments, in order to be able to establish if this would entail any differences in the risk and occurrence of thyroid dysfunction in the setting of treatment with ICPI. For instance, further functional tests of the serum lymphocytic profile,17,28 could probably better clarify whether hypothyroidism/thyroiditis more closely resembles sporadic Hashimoto's thyroiditis, maybe less probable given the current findings, or just a form of subacute thyroiditis. This could also be useful to differentiate patients who may be more likely to have persistent disease rather than a transient form of drug-induced thyroiditis. However, this is yet to be confirmed.
We did not find differences in baseline characteristics between patients who developed thyroid dysfunction and those who did not, in agreement with some previous studies.13 Although some studies pointed to a higher prevalence of endocrine IRAE in women, subsequent studies did not confirm these findings.14 Also, data specifically regarding thyroid autoimmunity or family history of thyroid alterations were not particularly informative, even though they were not routinely available for all patients. Whether assessment of thyroid antibodies is useful in identifying patients at risk of thyroiditis is not proven, nor if there could be a specific predisposition to immune checkpoint-related thyroid dysfunction in certain patients.14,31 In addition, reports on the follow-up of patients receiving these immune treatments are not usually evaluated for prior autoimmune diseases, autoimmune susceptibility or autoimmune family history, which may encumber a full comprehension of the underlying causes.
In any case, the clinical relevance and prognostic factor of these alterations still remain to be elucidated.5 In fact, in our cohort, hyperthyroid patients returned to normality with no need of an active intervention, and hypothyroid patients were easily managed with hormone replacement treatment. Regarding tumor outcome, some reports have described that patients who develop adverse events from ICPI are the ones whose cancers are most likely to respond to them,12,13,32 remarking the importance of maintaining a high degree of suspicion and an early management, in order to avoid the need for discontinuing cancer treatment because of an endocrine disorder that we should be relatively easy to manage. In our series, there were no significant differences in tumor outcome between patients who developed thyroid alterations and those who did not, and nivolumab was only withdrawn in one patient due to thyroid and pancreatic toxicity. So, although we provide more case descriptions to the literature, further prospective long-term studies are necessary to be able to address this issue.
We did not identify any case of hypophysitis, in agreement with previous reports, in which this endocrine alteration is more frequently described after ipilimumab, rather than after nivolumab.9,10,20 However, it is possible that, in a similar way as the thyroid dysfunction observed in our cohort, the clinical presentation of hypophysitis is often asymptomatic or non-specific, occult or subclinical, and may be masked by the use of exogenous steroids or, again, an overlap with cancer-related constitutional symptoms. Routine measurements of pituitary hormones may be profitable to overcome the potential underestimation of the true incidence of hypophysitis.17 In the few patients of our cohort in which random pituitary hormones were determined, results were normal, except for the patient with secondary hypogonadism, which could have possibly been multifactorial. Some have proposed the routine measurement of pituitary autoantigens.33 However, further prospective studies are still needed to investigate and validate the applicability of this praxis and verify if an active search is truly cost-effective.
We did diagnose a patient with autoimmune diabetes in addition to his thyroiditis. This has been less reported in the literature,34–37 but we remark the importance of being aware of this potential complication. In addition to pancreatic autoimmunity, analysis of HLA subtypes may aid an early identification of potential high-risk patients. Central hypogonadism has also been described in the setting of hypophysitis after treatment with ICPI.9,14 However, in our patient, prior testosterone values were not available, so the potential influence of sickness or age-induced hypogonadism cannot be ruled out. Therefore, once again, a specific evaluation and follow-up may be useful to allow an early diagnosis, and avoid overlooking subclinical endocrine non-thyroidal dysfunctions.
The duration of follow-up for screening of potential endocrine IRAEs has still not reached a consensus. In our series, thyroid dysfunction and the unique case of autoimmune diabetes occurred shortly after the initiation of nivolumab. However, hypophysitis was not detected after a short-term follow-up. This is in agreement with previous reports, in which endocrine IRAEs occurred usually after 2–4 cycles of nivolumab therapy, with a range of 5–36 weeks.9,14 However, Ryder et al.17 have reported delayed toxicities as long as 3 years after therapy, suggesting that monitoring should be more frequent and of longer duration.
We cannot ignore the limitations of our study. For instance, although our analysis was comprehensive and performed at a specialized single center, the retrospective nature of the review and the limited meticulous endocrine evaluations in the absence of clinical signs or symptoms in many patients represent challenges in accurately diagnosing endocrine IRAEs. We may have underestimated the true prevalence of other endocrine adverse events in our series, but it may be overturned given the fact that ECOG performance status did not significantly change and there were no clear signs or symptoms suggestive of new endocrine disorders.
In conclusion, we describe a large cohort of patients who received treatment for nivolumab as, at least, a second-line therapy for NSCLC in a single center, and comprehensively describe the occurrence of thyroid alterations, which may be more frequent than initially described. The relevance of these alterations deserve further investigation and functional lymphocytic tests may help elucidate the underlying mechanisms involved.
Compliance with ethical standardsThis study was approved by the Ethics Committee of our hospital (Hospital Universitario La Princesa). All procedures performed were in accordance with the ethical standards of our institutional committee (the Ethics Committee of Hospital Universitario La Princesa), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Although this was a retrospective study, and a formal consent would not be required, all patients signed a written informed consent before starting treatment in which it was specified that clinical and analytical data collected before and during follow-up could be potentially used in an anonymous way for investigation and publication.
Conflict of interestThe authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FundingThis research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contributionARL contributed to study conception and design, followed-up patients, researched, analyzed and interpreted data and wrote the manuscript. JR, JMST and RC followed-up patients, interpreted data and reviewed and edited the manuscript. MM contributed to study conception and design and reviewed and edited the manuscript. All authors contributed to the final version of this manuscript.