This study was intended to assess the influence of the rs16147 variant of the NPY gene on liver histology in patients with non-alcoholic fatty liver disease (NAFLD).
Material and methodsEighty-nine patients with NAFLD were recruited into the study. Serum chemistry tests were done including lipid profile, transaminases, adipokines, and insulin resistance. Genotype of polymorphism (rs161477) of the NPY gene was studied.
ResultsTwenty-three patients (25.0%) had the GG genotype (wild type) and sixty-six patients (75%) the GA (n=39) or AA (n=27) (mutant) types. Patients with A allele had a lower percentage of lobular inflammation and steatohepatitis (lobular inflammation plus ballooning) than those with wild genotype. Patients with A allele showed lower SAF (Steatosis, Activity, Fibrosis) scores than non-A allele carriers (5.4±2.7 points vs. 4.1±1.1 points; p=0.01). In the analysis without fibrosis (NAS score), the same differences were detected (4.5±1.8 points vs. 3.4±1.8 points; p=0.01). In the logistic regression analysis A allele carriers showed lower odds for inflammation (OR 0.11, 95% CI 0.02–0.84, p=0.03) and steatohepatitis (OR 0.39, 95% CI 0.14–0.86, p=0.04) after adjusting for age, sex, and body mass index.
ConclusionsA variant of polymorphism rs16147 of the NPY gene is independently associated to a lower percentage of steatohepatitis and lobular inflammation in obese subjects with biopsy-proven NAFLD.
El objetivo de nuestro estudio fue estudiar la influencia de la variante rs16147 del gen NPY en la histología hepática en pacientes con enfermedad de hígado graso no alcohólico (NAFLD).
Material y métodosSe reclutó una muestra de 89 pacientes con NAFLD. Se realizó un análisis bioquímico del suero que incluyó perfil lipídico, transaminasas, adipoquinas y resistencia a la insulina. Se estudió el genotipo de polimorfismo (rs161477) del gen NPY.
ResultadosUn total de 23 pacientes (25,0%) presentaron el genotipo GG (genotipo salvaje) y 66 pacientes (75%) GA (n=39) o AA (n=27) (genotipo mutante). Los pacientes con alelo A presentaron un menor porcentaje de inflamación lobulillar y esteatohepatitis que los pacientes con genotipo salvaje. Los pacientes con alelo A mostraron una puntuación más baja de SAF (esteatosis, actividad, fibrosis) que los no portadores de alelos A (5,4±2,7 puntos vs. 4,1±1,1 puntos, p=0,01). En el análisis realizado sin fibrosis (solo puntaje NAS), también se detectaron las mismas diferencias (4,5±1,8 puntos vs. 3,4±1,8 puntos, p=0,01). En el análisis de regresión logística a presencia del alelo A a una menor probabilidad de presentar con inflamación lobulillar (OR 0,11, IC 95%: 0,02-0,84; p=0,03) y esteatohepatitis (OR 0,39, IC 95%: 0,14-0,86, p=0,04) después de ajustar por edad, sexo e índice de masa corporal.
ConclusionesLa variante del polimorfismo rs16147 del gen NPY se asocia de forma independiente con un menor porcentaje de esteatohepatitis e inflamación lobulillar en sujetos obesos con NAFLD diagnosticado con biopsia.
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease.1 NAFLD is an epidemic metabolic liver disease in a lot of countries.2 This liver entity is characterized by accumulation of fat in the liver that could be accompanied by inflammation and necrosis resembling alcoholic hepatitis in the absence of heavy alcohol consumption.3 The rate of NAFLD is strongly linked to obesity, insulin resistance and metabolic syndrome.4 Liver tissue takes up circulating free fatty acids and low-density lipoproteins. Consistently, lipid storage contributes substantially to the liver triglyceride pool and liver steatosis.5 Its spectrum varies from simple steatosis to nonalcoholic steatohepatitis (NASH), which may progress to cirrhosis.6 In spite of high prevalence of NAFLD, little is known about its pathogenesis. Recent studies suggest that both environmental and genetic factors are involved in the progression and development of NAFLD.
Neuropeptide Y (NPY) is a 36-amino acid peptide neurotransmitter. It is expressed by chromaffin cells as well as noradrenergic cells.7 It has been proposed that increased NPY signaling due to high NPY expression in the hypothalamus contributes to the development of diabetes mellitus type 2, obesity and insulin resistance.8–9 The NPY gene has four exons and it is located at 7p15.1. The main genetic variant in this gene is rs16147 (G-399A).10 This genetic variant is associated with changes in NPY expression. According previous studies might be responsible for more than half of the variation in the expression of NPY in vivo.11 The association between NPY rs16147 SNP and metabolic syndrome (MetS) has seldom been reported. To our knowledge, only one previous have explored the importance of rs16147:T>C for regulation of NPY gene expression and brain function in 314 young adults.12 Recently, receptors of NPY have beed related to NAFLD fibrosis. Of note, in vitro study has demonstrated a reduced expression of Y1, Y4 and Y5 NPY receptors, whereas patients with NAFLD and advanced fibrosis (F4 or cirrhosis) had high expression of Y1, Y4 and Y6 NPY receptors in the liver of patients with NAFLD without fibrosis.13 Given the limited information about the relation of the polymorphisms of NPY gene with NAFLD, we aimed to evaluate the influence of polymorphism rs16147 of NPY gene on liver histology and biochemical parameters in patients with obesity and NAFLD.
Subjects and methodsSubjectsThe study group consisted of eighty nine Caucasian subjects with obesity. Participants were selected according to the following inclusion criteria: body mass index>30kg/m2, persistently elevated liver enzyme (ALT alanine amino transferase>40UI/L or AST aspartate amino transferase>31UI/L) with an abdominal ultrasound showing fatty liver. Exclusion criteria were significant alcohol consumption (>30g/day in males and >20g/day in females), hepatitis B, C, cytomegalovirus, Epstein Barr infections, non-organ-specific autoantibodies, diabetes mellitus, medication (blood-pressure lowering medication and statins), hereditary defects (iron and copper storage diseases and alpha 1-antitrypsin deficiency) and medication that might induce steatosis (glucocorticoids, estrogens, tamoxifen or valproic acid). All participants signed an informed consent. This protocol was conducted according to the guidelines laid down in the Declaration of Helsinki, the local ethics committee (HCUVA) approved all procedures involving patients and patient data were codified to guarantee anonymity.
Liver histologyThe diagnosis of NAFLD was confirmed in all patients by percutaneous liver biopsy performed with a Menghini-type biopsy needle. The biopsy site was located in the seventh or eighth intercostal space in the mid-axillary line. The site was further confirmed with ultrasonography. Skin, subcutaneous tissue, muscles, and peritoneum were infiltrated with the local anesthetic (procainamide 1%, 1ml at most). Vim–Silverman needle (short trocar and cannula) was introduced until the needle reached the liver. Then, trocar was removed and the 1cm long biopsy punched out by the introduction of a longer cannula split longitudinally that turned around to cut the liver piece. Biopsied liver was withdrawn through the cannula. Liver samples were sectioned, and stained with hematoxylin–eosin and Manson's trichrome. NAFLD was defined histologically by the presence of minimum 5% of steatosis on liver biopsy. Steatosis was scored as 1 (5–33%), 2 (34–66%), or 3 (>66%). Lobular inflammation was scored as 0 (presence of no inflammation), 1 (<2 foci per 200× field), 2 (2–4 foci per 200× field), or 3 based on >4 foci per 200× field. Ballooning was scored as 0 (no balloon cell), 1(few balloon cells), and 2 (many cells/prominent ballooning cells). A case presenting with at least grade 1 of each of the two features (ballooning, and lobular inflammation) and steatosis (NAS score) was classified as non alcoholic steatohepatitis (NASH).14 Fibrosis was scored as 0 (no fibrosis), 1 (perisinusoidal or periportal), 2 (perisinusoidal and portal/periportal), 3 (bridging) and 4 (cirrhosis). To minimize inter-observer variability, liver biopsy specimens were read by the same pathologist using the SAF (Steatosis, Activity, Fibrosis) score which assesses the grade of steatosis (S, from S0 to S3), the grade of activity (A from A0 to A4 by adding grades of ballooning and lobular inflammation, both from 0 to 2) and the stage of fibrosis (F from F0 to F4).14
Genotyping and biochemical parametersDNA was extracted using commercial kit extraction (Biorad, LA, CA). Primers were designed with the Sequenom Assay Design v4 (SEQUENOM, Inc., San Diego, CA). Genotyping for the rs16147 polymorphism was performed by chain reaction real time analysis. This polymerase chain reaction (PCR) was carried out with 20–25ng of genomic DNA, 0.1–0.15μl each of oligonucleotide primer for rs16147 (primer forward: 5′-ACGTTGGATGCACAAAGAGGATTCAGGTGC-3′ and reverse 5′-ACGTTGGATGAGCCCAGACGATTCTTGTC-3′ in a 2-μl final volume (Termociclador Lifetecnologies, LA, CA). DNA was denatured at 85°C for 5min; this was followed by 45 cycles of denaturation at 95°C for 15s, and annealing at 58.1°C for 45s). The PCRs were run in a 2-μl final volume containing 0.1μl of iPLEx Termination mix (Bio-Rad®, San Diego, CA) with hot start Taq DNA polymerase. Hardy Weinberg equilibrium was calculated with a statistical test (Chi-square). The variant of NPY gene was in Hardy Weinberg equilibrium (p=0.21).
Blood samples were collected in Na-EDTA tubes from patients after 12h fast. All samples were frozen at −80°C until laboratory testing. Insulin was assessed by radioimmunoassay (RIA) (RIA Diagnostic Corporation, Los Angeles, CA) with a sensitivity of 0.5mUI/L (normal range 0.5–30mUI/L)15 and the homeostasis model assessment for insulin resistance (HOMA-IR) was calculated using the next formula (fasting insulin×fasting glucose concentrations/22.5).16 Plasma glucose levels, total cholesterol, triglyceride concentrations, HDL cholesterol, alanine amino transferase, aspartate aminotransferase activity and Gama glutamine transferase were measured by enzymatic colorimetric assay Hitachi 902 analyser (Hitachi Ltd., Tokyo, Japan). LDL cholesterol was calculated using Friedewald formula.17
Resistin was measured by Enzyme-Linked Immunoabsorbent Assay (ELISA) (Biovendor Laboratory, Inc., Brno, Czech Republic) with a sensitivity of 0.2ng/ml and a normal range of 4–12ng/ml.18 Leptin was measured by ELISA (Diagnostic Systems Laboratories, Inc., Texas, USA) with a sensitivity of 0.05ng/ml and a normal range of 10–100ng/ml.19 Adiponectin was measured by ELISA (R&D systems, Inc., Minneapolis, USA) with a sensitivity of 0.246ng/ml and a normal range of 8.65–21.43ng/ml.20
Anthropometric measurementsBody weight was assessed to an accuracy of 10g and body mass index calculated as body weight in kg/(height in m2). Waist and hip circumferences to derive waist-to hip ratio (WHR) were measured, too. Bioimpedance was used to determine total body fat mass with an accuracy of 10g21 (Akern, EFG, It). Resistance and reactance were used to calculate total body water, fat and fat-free mass.
Statistical analysisSample size was calculated to detect a 15% of differences in percentage of steatohepatitis between genotype groups in a dominant model. Data are expressed as mean±standard deviation or as frequencies for categorical variables. Categorical variables were analyzed with the Chi-square test, with Yates correction as necessary, and Fisher's test. Continuous variables with normal distribution were analyzed with a two-tailed Student's t-test. Non-parametric variables were analyzed with the Mann–Whitney U test. Logistic regression analysis was used to test the association of rs16147 with liver histology (steatosis, fibrosis, ballooning and lobulillar inflammation as independent dichotomy variables, presence vs. absence) adjusted by sex, age, waist circumference and BMI. It was calculate OR and 95% confidence interval (CI). The genotype distribution was tested for deviation from Hardy–Weinberg equilibrium by a Chi-square test with 1 df (p>0.05). The statistical analysis was performed for the combined GA and AA as a group and GG genotype as second group, with a dominant model. The statistical package was SPSS 15.0 (IL, USA). A p-value under 0.05 was considered statistically significant.
ResultsEighty nine patients gave informed consent and were enrolled in the study. The mean age was 45.1±8.5 years and the mean body mass index (BMI) 37.2±7.0kg/m2). All subjects were weight stable during the 12 weeks period preceding the study (body weight change, 0.16±0.1kg).
Thirty two were men (35.2%) and 57 women (64.8%). Twenty three patients (25.0%) had the genotype GG (wild type group) and sixty six patients (75%) patients GA (n=39) or AA (n=27) (mutant type group). The allelic frequency of A was 0.53. Age and gender distribution were similar in all groups.
Table 1 details the anthropometric variables. No differences were reported between both genotype groups.
Anthropometric variables.
| Characteristics | GG (n=22) | (GA or AA) (n=66) |
|---|---|---|
| BMI (kg/m2) | 37.8±9.3 | 37.9±9.1 |
| Weight (kg) | 129.8±31.7 | 126.5±23.1 |
| WC (cm) | 122.7±12.2 | 119.7±1.5 |
| Fat mass (kg) | 37.8±11.1 | 36.9±8.2 |
BMI: body mass index. WC: waist circumference. No statistical differences between groups. Parameters expressed in (mean±standard deviation).
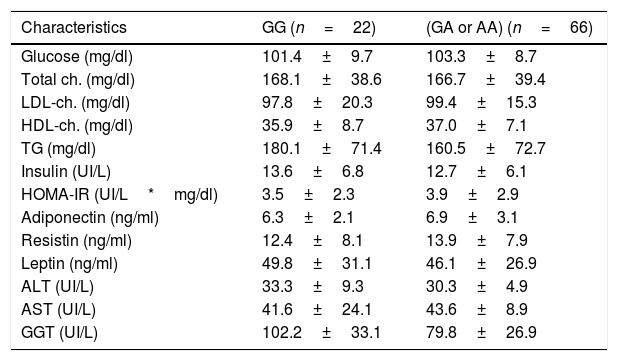
Table 2 details the classic biochemical parameters. Lipid profile, levels of adipokines, liver biochemistry and glucose metabolism did not show statistical differences.
Biochemical parameters.
| Characteristics | GG (n=22) | (GA or AA) (n=66) |
|---|---|---|
| Glucose (mg/dl) | 101.4±9.7 | 103.3±8.7 |
| Total ch. (mg/dl) | 168.1±38.6 | 166.7±39.4 |
| LDL-ch. (mg/dl) | 97.8±20.3 | 99.4±15.3 |
| HDL-ch. (mg/dl) | 35.9±8.7 | 37.0±7.1 |
| TG (mg/dl) | 180.1±71.4 | 160.5±72.7 |
| Insulin (UI/L) | 13.6±6.8 | 12.7±6.1 |
| HOMA-IR (UI/L*mg/dl) | 3.5±2.3 | 3.9±2.9 |
| Adiponectin (ng/ml) | 6.3±2.1 | 6.9±3.1 |
| Resistin (ng/ml) | 12.4±8.1 | 13.9±7.9 |
| Leptin (ng/ml) | 49.8±31.1 | 46.1±26.9 |
| ALT (UI/L) | 33.3±9.3 | 30.3±4.9 |
| AST (UI/L) | 41.6±24.1 | 43.6±8.9 |
| GGT (UI/L) | 102.2±33.1 | 79.8±26.9 |
LDL: low density lipoprotein. HDL: high density lipoprotein. Ch: cholesterol. TG: triglycerides. HOMA-IR: homeostasis model assessment. ALT: alanine amino transferase. AST: aspartate amino transferase. GGT: gamma glutamil transferase. No statistical differences between groups. Parameters expressed in mean±standard deviation.
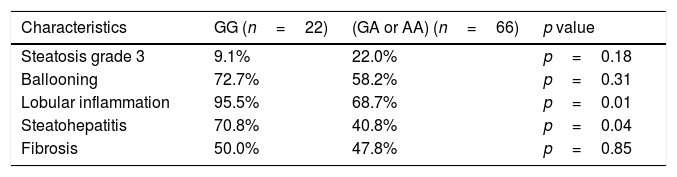
Table 3 shows the histological findings according genotype. Patients with A allele presented a lower percentage of lobulillar inflammation and steatohepatitis (lobulillar inflammation plus ballooning) than patients in the wild type group. Patients with A allele showed lower SAF score than non-A allele carriers (5.4±2.7 points vs. 4.1±1.1 points; p=0.01). In the analysis of this score performed without fibrosis (NAS score), the same differences were also detected (4.5±1.8 points vs. 3.4±1.8 points; p=0.01).
Histological parameters (frequencies).
| Characteristics | GG (n=22) | (GA or AA) (n=66) | p value |
|---|---|---|---|
| Steatosis grade 3 | 9.1% | 22.0% | p=0.18 |
| Ballooning | 72.7% | 58.2% | p=0.31 |
| Lobular inflammation | 95.5% | 68.7% | p=0.01 |
| Steatohepatitis | 70.8% | 40.8% | p=0.04 |
| Fibrosis | 50.0% | 47.8% | p=0.85 |
Steatosis grade 3 (> 66%).
Chi square test, *p<0.05 (%) frequencies in each genotype group.
Finally, logistic regression analysis indicated that subjects with A-allele carriers presented lower probability of lobulillar inflammation (OR 0.11, 95% CI 0.02–0.84, p=0.03) and steatohepatitis (OR 0.39, 95% CI 0.14–0.86, p=0.04) after adjusting by age, sex and BMI. If the BMI is replaced by the waist circumference, the adjustment models are not modified.
DiscussionThe main finding of this cross-sectional study was the beneficial effect of A allele on liver histology. This effect was independent of anthropometric and biochemical anthropometric and biochemical parameters. Carriers of A allele exhibited lower percentage of steatohepatitis and lobulillar inflammation than non A allele carriers.
The NPY gene is highly polymorphic, some SNPs (single nucleotide polymorphism) of this gene have been associated with obesity and cardiovascular risk in some22–23 but not all studies.24 The differences of these studies with our design is important; firstly, the population of the previous studies is no a Caucasian population and secondly, the inclusion criteria in some studies are very different from ours. For example, patients of this study24 had a documented coronary artery disease; however the patients in our study had no coronary disease. The age of the populations studied can also be an important factor in the findings found in the literature. For example, Zain et al.25 reported that A allele of rs16147 is associated with increased risk of obesity, whereas A allele of rs5574 is associated with a reduced risk of obesity. Olza et al.26 reported similar results with rs16147 in a pediatric population. Hohmann et al.27 reported a positive an increasing association between NPY promoter polymorphism and BMI during the course of development child growth, too. However, all these findings have not been replicated in the adult population.28,29 Finally, the samples evaluated are not comparable, and in the particular case of our population of obese adults with cardiovascular risk, other genetic and environmental factors may be involved in our findings.
To our knowledge, this research is the first to study the effect of this polymorphism of the NPY gene on liver histology in NAFLD subjects. Our results show that the A allele influence on lobulillar inflammation and steatohepatitis independently of body weight, age and sex. A previous study evaluating the NPY pathway has shown that patients with NAFLD presented a positive correlation between saturated fatty acid intake and NPY levels.30 Perhaps an interaction between the dietary intake in our study participants and the NPY pathway could explain our histopathological findings. On the other hand, in our study did not observe between a relationship between adipokine levels and this SNP. Nevertheless, a negative correlation between NPY and adiponectinemia in NAFLD obese adolescents has been reported.31 Mutschler et al.27 reported an interaction of this SNP with leptin levels in females, too.
The precise molecular mechanisms underlying our observed association between rs16147 and liver histology are unclear. Molecular studies have been developed to NPY after its discovery as an orexigenic neuropeptide, since those initial studies, NPY has also been found to affect energy expenditure independent of food intake.32 Perhaps this effect on energy expenditure can influence the accumulation of fat at the body and specifically in the liver tissue at the liver level. Another potential hypothesis is the level of expression of different subtypes of NPY receptors in the liver at the hepatic level. Sigala et al.13 have reported a relationship between expression of different types of NPY receptors and liver fibrosis. Since NAFLD patients without fibrosis had reduced expression of Y1, Y4 and Y5 NPY receptors, whereas patients with NAFLD with cirrhosis had high expression of Y1, Y4 and Y6 NPY receptors in an in vitro study.13 Other in vitro study33 has shown the proliferative effects of NPY on hepatic stellate cells.
A strength of our study, it is the use of liver biopsy as a diagnostic method for NALFLD. Our study has some limitations. First, we did not measure serum NPY levels in order to evaluate their direct effects on hepatic histology. Secondly, there are uncontrolled non-genetic factors such as exercise, hormone levels, and other lifestyle factors that could influence the relationships observed in our study, none of which were taken to account in the present study. For example, different studies of dietary intervention34,35 have shown that the metabolic response to diet is modulated depending on the presence of the allele A. Thirdly, other variants of this gene or other genes that are in imbalance can influence the results obtained in our findings. Fourthly, the difficulties in generalizing the results to other populations of patients with non-alcoholic fatty liver disease, such as patients without significant obesity (the mean BMI of the included patients was >35kg or with diabetes (comorbidity frequently associated with NAFLD). Finally, our study cannot prove causality due to its cross-sectional design.
In conclusion, A variant of the polymorphism rs16147 of NPY gene is independently associated with a lower percentage of steatohepatitis and lobulillar inflammation in obesity subjects with proven biopsy NAFLD. Therefore, future studies are necessary to find out if it is necessary to evaluate different polymorphisms in the patient with NAFLD at the time of diagnosis and before initiating the treatment.
FundingThere is no financial support.
Conflict of interestThere is no conflict of interest.