We report the case of a 48-year-old man with a history of familial colonic polyposis and iron deficiency secondary to gastrointestinal losses being treated with intravenous iron therapy (ferric carboxymaltose; Ferinject®), as well as psoriasis and polyarthritis. He was referred from rheumatology for calcium and phosphorous metabolism laboratory testing, yielding the following values: albumin-corrected calcium 8.1 mg/dl (8.4–10.2), phosphate (P) 1.1 mg/dl (2.5–4.5), 25-hydroxy vitamin D 31 ng/ml, intact parathyroid hormone (PTH) 71.5 pg/ml (15–65) and urinary phosphate excretion 35% (10%–20%). In light of these findings, which were consistent with abnormal phosphorus metabolism, further laboratory testing prior to an in-person appointment was ordered consisting of calcitriol and fibroblast growth factor 23 (FGF-23). The results were: albumin-corrected calcium 8.06 mg/dl, phosphate 1 mg/dl, 25-hydroxy vitamin D 32.4 ng/ml, renal phosphate excretion (RPE) 60%, intact PTH 82.4 pg/ml, calcitriol 44 pg/ml (20–54), (FGF-23) 93.4 RU/ml (as a guideline up to 145). Inappropriately high FGF-23 levels and inappropriately low calcitriol levels for blood phosphate levels pointed to hypophosphataemia due to FGF-23–dependent renal losses. Treatment was then started with 0.5 mcg of calcitriol daily for seven days, followed by phosphate salts with 799 mg of elemental phosphorus every eight hours on a diet with normal calcium intake (two daily portions of dairy products). Subsequent check-ups found normalisation of serum levels of phosphate, calcium and intact PTH, as well as urinary phosphate excretion; two months after treatment was started, the patient's laboratory values were as follows: albumin-corrected calcium 8.9 mg/dl (8.4–10.2), phosphate 2.5 mg/dl (2.5–4.5), PTH 58.7 pg/ml (15–65) and RPE 20%.
Aetiologically, the patient was found to be on ongoing treatment with ferric carboxymaltose every three months ever since March 2016 (dose: 500−1,000 mg IV) due to iron deficiency secondary to losses in the context of his colon disease (he had declined to undergo a total colectomy). Intravenous compounds were developed to improve response to oral treatment in cases of intolerance or lack of capacity for oral absorption. Four notable such formulations (ferric carboxymaltose, ferric derisomaltose [or iron isomaltoside], ferumoxytol and low-molecular-weight iron dextran) achieve fast and effective iron replacement.
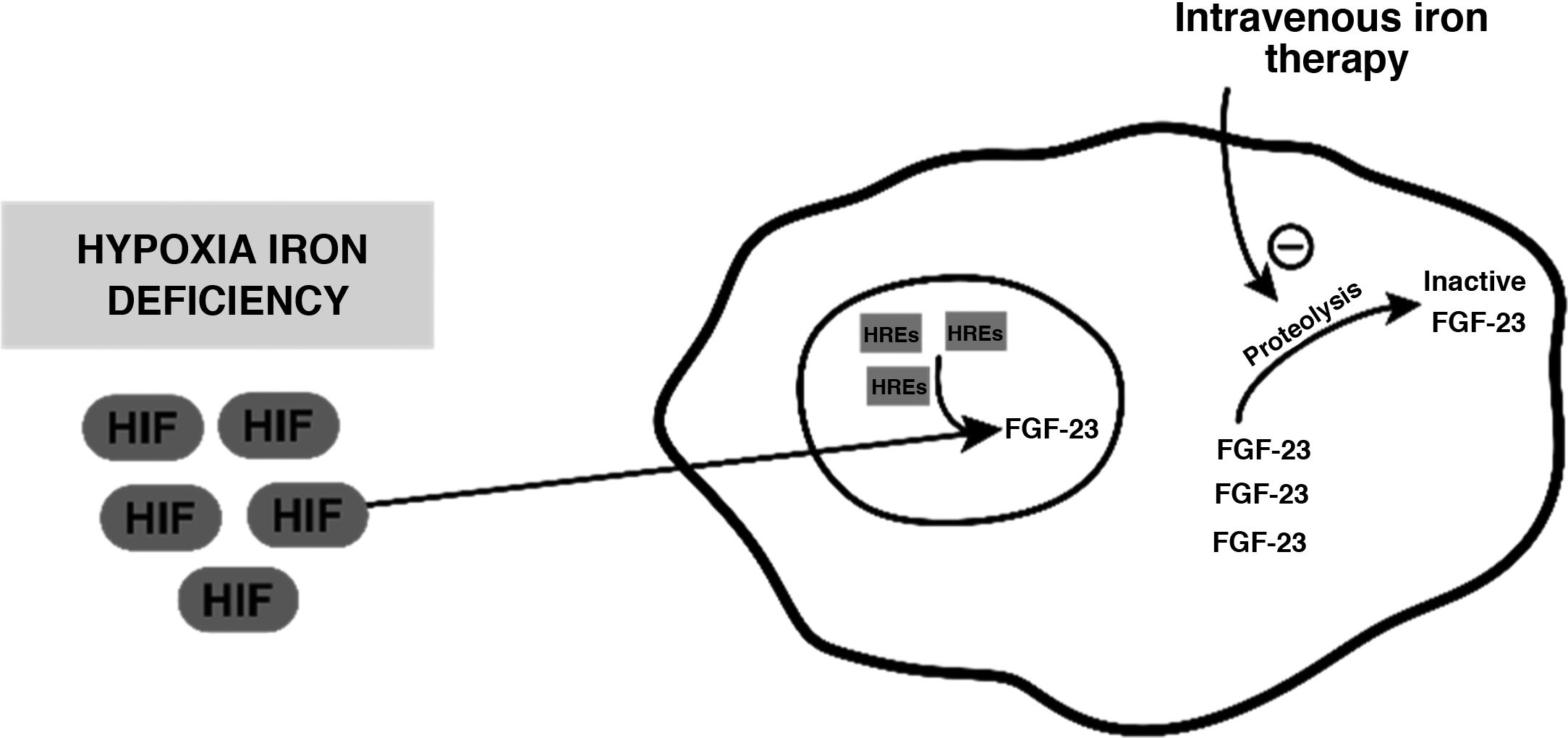
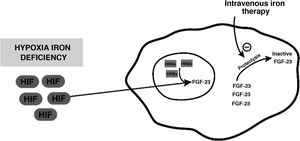
Despite its safety profile, intravenous iron therapy is not free from adverse effects. One of them is secondary hypophosphataemia, whose mechanism of action is postulated to be a result of an increase in FGF-23 levels. Iron deficiency per se does not cause hyperphosphaturic hypophosphataemia; it does cause an increase in production of an inactive form of hypoxia-induced FGF-23; this is found to be mediated by hypoxia inducible factor, which translocates to the cell nucleus and, along with hypoxia response elements (HREs), produces inactive forms through proteolytic cleavage thereof. Intravenous infusion of iron would act by inhibiting this cleavage and thus causing an increase in active FGF-23 levels1–3 (Fig. 1).
Diagram representing the mechanism postulated through which inactive FGF-23 levels increase, in the event of hypoxia or iron deficiency, and how intravenous iron therapy would act on this level.
FGF-23: fibroblast growth factor 23; HIF: hypoxia inducible factor; HREs: hypoxia response elements.
However, not all compositions cause hypophosphataemia to the same degree or by the same mechanism; various comparative studies have demonstrated a higher frequency in patients administered ferric carboxymaltose.
Two randomised clinical trials compared the incidence between ferric carboxymaltose and iron isomaltose. Both Trial A (n: 123; 62 iron isomaltoside and 61 carboxymaltose) and Trial B (n: 122; 61 iron isomaltoside and 61 carboxymaltose) yielded lower incidences of hypophosphataemia in patients treated with isomaltose.4
Another trial compared the incidence of hypophosphataemia in a group administered ferric carboxymaltose (n: 1000) versus ferumoxytol (n: 997) and found a significantly higher incidence in the first group (for P levels <2 mg/dl 50.8% versus 0.9%; for P levels <1.3 mg/dl 10% versus 0.0%; p < 0.001). In addition to incidence, a subgroup was prepared to analyse the different biochemical mediators involved with 98 patients treated with carboxymaltose and 87 patients treated with ferumoxytol, revealing that in the first group active FGF-23 levels increased, whereas in the second group they were not elevated. This elevation of active FGF-23 was significantly associated with hypophosphataemia, hyperphosphaturia and a drop in calcitriol.2 Hypophosphataemia can occur as of administration of the first dose of intravenous iron. In most cases, hypophosphataemia associated with intravenous iron therapy is asymptomatic and transient. However, repeated doses or prolonged administration can render it serious and/or symptomatic. The risk of developing symptoms is directly proportionate to hypophosphataemia severity and recovery time.1 The most common symptoms are asthenia, fatigue, bone pain and/or fractures, as well as other bone abnormalities, myopathies which may be associated with secondary rhabdomyolysis and even respiratory arrest.1 Treatment is essentially based on expert experience and opinion; there is no consensus. There is an agreement on using it based on clinical signs, hypophosphataemia severity and underlying cause. Phosphorus supplements are usually used; these can be administered orally or intravenously, in addition to support therapies that help improve intestinal absorption of P, such as vitamin D correction. Considering the effects of FGF-23 on vitamin D metabolism, calcitriol is recommended.1 In refractory cases, in the first documented and published case of the use of burosumab (a human monoclonal antibody that targets FGF-23, approved to treat X-linked hypophosphataemia), a patient with FGF-23-mediated osteomalacia induced by intravenous iron therapy showed clinical, laboratory and radiological improvement as of the first administration of the drug.5
Intravenous iron administration represents a safe and effective means of replenishing deficient iron stores; however it is not free from adverse reactions. In this context, hypophosphataemia is reported in the scientific literature, but remains unknown to many physicians. Although it is normally an asymptomatic and transient condition, given the widespread and growing use of intravenous iron therapy and the fact that it can sometimes cause significant complications, it would be advisable to monitor the patient for this condition during said therapy.