Obesity may be present in patients with primary hypothalamic lesions, particularly craniopharyngioma (CP), but it is also the most common complication after its surgical treatment.1–4 The hypothalamic nuclei, responsible for appetite and basal metabolism, are implicated in its pathogenesis.5,6 There is currently no effective treatment.5 We report the case of a patient with hypothalamic obesity following surgery for CP who responded to treatment with a glucagon-like peptide-1 (GLP-1) analogue.
The patient, a 30-year-old woman referred from another hospital, had undergone surgery for CP at the age of 11 years and required another two surgical procedures and radiation therapy. She had panhypopituitarism with primary amenorrhea, daytime somnolence, and slight intellectual impairment. Patient complained of excess appetite and episodes of binge eating. Shortly after surgery, she was diagnosed with diabetes mellitus, and insulin therapy was started. She is on hormone replacement therapy with levothyroxin 200mcg/day, hydrocortisone 25mg/day in three divided doses (15mg–5mg–5mg), antidiuretic hormone (six intranasal applications daily), transdermal patches with 600mcg of ethinylestradiol/6mg of norelgestromin (one weekly patch three weeks per month). Antidiabetic treatment consists of metformin/vildagliptin 1000/50mg/12h, insulin detemir 12IU/12h, and insulin glulisine as needed (5–10IU with each meal). Her blood glucose control has always been poor, with hypoglycemic episodes and obesity. Patient is also being treated with valproic acid 1000mg/12h, topiramate 100mg/12h, perampanel 18mg/24h, fluoxetine 40mg/24h, and levetiracetam 1000mg/12h. Physical examination found a height of 161cm, 88kg of weight (body mass index 34), sexual infantilism with telarche but no adrenarche, and mild diabetic retinopathy.
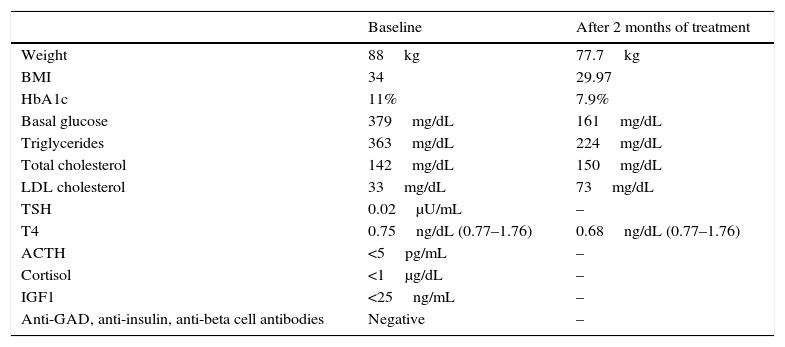
Table 1 shows her relevant laboratory data. We decided to modify treatment by adjusting levothyroxin to 225mcg/day, changing to insulin degludec 19IU at night and metformin 1000mg/12h, and adding dulaglutide 1.5mg one application each week. Start of growth hormone treatment was delayed because of poor blood glucose control at the time and presence of diabetic retinopathy.
Physical examination and laboratory data of the patient during follow-up.
| Baseline | After 2 months of treatment | |
|---|---|---|
| Weight | 88kg | 77.7kg |
| BMI | 34 | 29.97 |
| HbA1c | 11% | 7.9% |
| Basal glucose | 379mg/dL | 161mg/dL |
| Triglycerides | 363mg/dL | 224mg/dL |
| Total cholesterol | 142mg/dL | 150mg/dL |
| LDL cholesterol | 33mg/dL | 73mg/dL |
| TSH | 0.02μU/mL | – |
| T4 | 0.75ng/dL (0.77–1.76) | 0.68ng/dL (0.77–1.76) |
| ACTH | <5pg/mL | – |
| Cortisol | <1μg/dL | – |
| IGF1 | <25ng/mL | – |
| Anti-GAD, anti-insulin, anti-beta cell antibodies | Negative | – |
ACTH: adrenocorticotropic hormone; HbA1c: glycosylated hemoglobin; IGF-1: insulin-like growth factor 1; BMI: body mass index; LDL: low density lipoprotein; TSH: thyroid-stimulating hormone; T4: T4-levothyroxin.
After two months, patient reported that a decreased appetite and absence of binge eating episodes or hypoglycemia. She was less drowsy. She had lost 10.3kg, and basal insulin dose had to be gradually tapered, while prandial insulin dose was decreased to 6–6–3 (Table 1). Levothyroxin was increased again to 250mcg/day due to persistently low T4 levels. On the third month, rapid insulin was discontinued based on capillary glucose levels.
Research conducted over the past few decades has shown that the body's energy homeostasis depends on: (1) infundibulo-tuberal nuclei; (2) peripheral tissues (white and brown adipose tissue); (3) autonomic nervous system; (4) hormonal and metabolic signals (insulin, glucocorticoids), and gastrointestinal tract signals.4,7
After surgery and radiation therapy for CP, patient experienced panhypopituitarism, infundibulo-tuberal syndrome (diabetes insipidus, somnolence, and adiposogenital dystrophy1,2), and diabetes mellitus. Infundibulo-tuberal syndrome is the result of damage to the arcuate, ventromedial, tuberal, and tuber cinereum nuclei.1,6 Additionally, despite the age of onset, diabetes mellitus was not caused in this patients by autoimmune pancreatic failure but more probably by damage to the arcuate nucleus, which has leptin receptors responsible for regulating glucose metabolism, modulating the function of the sympathetic nervous system.6
Obesity is common in patients with hypothalamic lesions (25%), but is rarely the first symptom. Obesity usually occurs when large lesions are present. Specifically, bilateral destruction of ventromedial nuclei causes obesity, and is due in 90% of cases to tumors, of which CP is most common (60% of cases).8 A 70% prevalence of obesity has been reported with this tumor. After surgery, obesity appears as a complication in 50% (30–77%) of patients in whom it did not exist before, half of them with severe hyperphagia.1,4,9,10
Four risk factors for development of obesity after treatment of brain tumors in children have been reported5: (1) tumor location in hypothalamus or thalamus; (2) diencephalic tumors; (3) direct radiation to hypothalamus; (4) concurrent hormone deficiency. All these circumstances, present in our patient, reflect hypothalamic damage. It has been postulated that topographic location of CP is the main risk factor for developing hypothalamic obesity. Hydrocephalus and associated endocrine diseases have some influence, but are not determinant.4,8 In our patient's specific case, growth hormone and thyroid hormone deficiencies may have contributed to occurrence of obesity. As regards the exact mechanism causing this obesity associated to hypothalamic damage, some authors propose an imbalance between the mechanisms of satiety and appetite. Patients with CP have been found to have a decreased energy expenditure, both basal and voluntary, even in cases with normal intake, and those in whom the tumor causes hypothalamic damage show a significant increase in body mass index. Interruption of response to anorexigenic signals such as leptin and insulin caused by hypothalamic damage is a possible mechanism for development of obesity. Hyperinsulinemia and autonomic nervous system imbalance may also be involved.6,9,10 Patients with diabetes insipidus who require chronic hormone replacement therapy have more marked obesity, possibly reflecting more severe damage, consisting of a bilateral lesion of both supraoptic and paraventricular nuclei caused by the tumor.9
Calorie restriction is of little value to treat hypothalamic obesity. Asthenia–which persists after appropriate hormone replacement–and the decreased physical activity seen in these patients lead to lower utilization of calorie provision.5,10
The drugs generally used for the treatment of obesity have not yet proved to be effective in these patients.4,10 GLP-1 analogues are antidiabetic drugs administered subcutaneously. They act at GLP-1 receptors located in the stomach, duodenum, and exocrine pancreas, as well as on various hypothalamic and diencephalic nuclei involved in appetite regulation.10,11 The effects of these drugs are satiety, due to their action on the central nervous system (particularly on the arcuate and paraventricular nuclei), a slowing down of stomach emptying, stimulation of insulin and glycogen production, and inhibition of glucagon production through their peripheral action.10,11 Administration of these drugs is associated to weight loss due to a combination of all these effects. Their use for treatment of hypothalamic obesity is not yet widespread. Cases of hypothalamic obesity, mostly secondary to CP or its treatment, have responded to treatment with daily GLP-1 analogues (exenatide and liraglutide).10 In some of these patients, treatment had to be discontinued due to side effects, especially nausea and vomiting. The exact mechanisms by which weight loss is achieved in these patients using GLP-1 analogues despite hypothalamic damage have not been elucidated yet.10 One possible explanation is that blood levels of the GLP-1 analogue reached are much higher than physiological GLP-1 levels, and could overcome a theoretical hypothalamic resistance to peripheral signals responsible for informing on the nutritional status of the body. Our patient was treated with dulaglutide, a weekly GLP-1 analogue, to simplify the treatment regimen. There has been a significant weight loss, lower requirement of insulin–it was even discontinued–and better blood glucose control. Patient reported satiety and disappearance of binge eating episode, and had no side effects. Her treatment was simplified, and her quality of life substantially improved.
To sum up, obesity of hypothalamic origin due to a primary lesion or occurring after surgery is common and difficult to manage. Lifestyle and diet interventions and appropriate hormone replacement are often insufficient. There is not enough experience with the drugs currently available for the treatment of obesity. We report the case of a patient with hypothalamic obesity secondary to treatment of CP who significantly improved with use of dulaglutide, a GLP-1 analogue.
FundingThere are no funding sources.
Conflicts of interestThe first author has occasionally collaborated with Lilly in training sessions.
Please cite this article as: Castro-Dufourny I, Carrasco R, Pascual JM. Obesidad hipotalámica tras intervención quirúrgica de un craneofaringioma: tratamiento con un análogo del péptido similar al glucagón tipo 1. Endocrinol Diabetes Nutr. 2017;64:182–184.