The BRAF V600E mutation is the most common genetic change in papillary thyroid carcinoma and is associated with a poorer clinical course. Usual methods for its study (DNA sequencing or molecular test based on PCR) are expensive and time-consuming. Recently, immunohistochemistry (IHC) for BRAF mutation has been introduced.
ObjectiveTo compare the results of IHC and real time PCR (RT-PCR) in the detection of BRAF V600E mutation in papillary thyroid carcinoma. Analysis of clinical and pathological differences depending on RT-PCR results is included.
MethodsA prospective study was performed in 82 consecutive samples, 54 of them taken through a core needle biopsy. IHC was performed on tissue fixed for 24h with 10% neutral formalin using the anti-BRAF V600E (VE-1) mouse monoclonal primary antibody and was rated as positive or negative. DNA was extracted from formalin-fixed, paraffin-embedded tissues by manual microdissection, and BRAF mutation was detected by RT-PCR using the Cobas® 4800 BRAF V600 mutation test (Roche).
ResultsBoth techniques were concordant in 81 cases, and BRAF was positive in 49. Discordance appeared in a follicular variant showing positive IHC and negative RT-PCR, attributed to histological heterogeneity. Cost of materials for IHC was less than half of the cost for RT-PCR.
ConclusionsIHC appears to be a reliable, economical and easily available alternative to molecular biology techniques for routine detection of the BRAF V600E mutation in papillary thyroid carcinoma patients, provided optimal fixation conditions are used. It may be a useful technique in hospitals with no access to molecular biology techniques.
La mutación V600E de BRAF (protooncogén B-Raf) asocia mayor riesgo de persistencia y recidiva en el carcinoma papilar de tiroides, y puede modificar la cirugía o el seguimiento. Las tecnologías de biología molecular empleadas en su detección son caras y técnicamente demandantes. Recientemente se ha propuesto la evaluación inmunohistoquímica (IHQ), más sencilla y asequible, que permitiría universalizar su evaluación.
ObjetivoComparar los resultados y el coste económico del estudio IHQ frente a la PCR en tiempo real (RT_PCR) para la detección de BRAF V600E en los carcinomas papilares de tiroides. Se incluyó el análisis de las diferencias clínico-patológicas según el resultado por RT_PCR.
MétodosEstudio prospectivo sobre 82 muestras consecutivas, 54 de ellas biopsias con aguja gruesa. El estudio IHQ se realizó con el anticuerpo monoclonal murino VE-1, y fue categorizado como positivo o negativo. La detección mediante RT_PCR se realizó con la prueba diagnóstica Cobas® 4800 (Roche) sobre ADN extraído del tejido fijado por microdisección manual.
ResultadosAmbas técnicas fueron concordantes en 81 casos (98,8%), con un resultado discordante, positivo en la IHQ y negativo en la RT_PCR, atribuido a heterogeneidad histológica. Solo en gasto de material diagnóstico, la IHQ logra un ahorro superior al 50% frente a la técnica molecular.
ConclusionesLa detección IHQ de la mutación BRAF V600E presenta una elevada fiabilidad, sin falsos negativos, en muestras adecuadamente procesadas. Su empleo permite abaratar costes y generalizar su empleo, especialmente en centros sin acceso rutinario a técnicas de biología molecular.
The BRAF V600E mutation (B-Raf proto-oncogene) is the most common genetic change in advanced papillary thyroid carcinoma (PTC); its prevalence ranges from 29% to 83% in various populations.1 Numerous studies have shown the mutation to be associated with aggressive clinicopathological characteristics2 and with greater disease recurrence.3 Because of this association, it has been considered a potential prognostic maker in PTC, and the recent American Thyroid Association guidelines4 include it as an indicator of additional risk in certain cancer subtypes. Its preoperative detection has also been used to establish a diagnosis of papillary carcinoma in indeterminate cytology samples.5
Several molecular methods to identify the mutation have been developed. The first of the two most commonly used procedures is direct sequencing of DNA amplified by the polymerase chain reaction (PCR) by the Sanger method, which uses the analysis of the different complementary DNA fragments generated by the incorporation of chain-terminating dideoxynucleotides. A commonly used variant is pyrosequencing, based on the detection of pyrophosphate release after nucleotide incorporation.
The second method is based on allele-specific PCR. Real-time PCR (RT-PCR) is most commonly used to identify molecular biomarkers in tumors.6,7 RT-PCR has high sensitivity (detects the mutation in samples with less than 10% of tumor cells), reproducibility, and automation capability. There are two types of probes to determine BRAF mutations, called TaqMan® and Scorpions®. The cobas®4800 (Roche) test, designed to determine the V600E mutation, uses the TaqMan® probe, and is approved by the Food and Drug Administration as an in vitro diagnostic test. The Scorpions® probe is used with the Qiagen® BRAF Rotor-Gene Q test. RT-PCR is expensive, labor-intensive, and time-consuming because it is performed in sequential steps. For this reason, its availability is limited.
A recently developed mutation-specific antibody (clone VE-1) allows for immunohistochemical (IHC) visualization of the mutated protein with high sensitivity and specificity VE-1 is a mouse monoclonal antibody that has proved to be reliable in several tumor tissues, including thyroid cancer8 and melanoma.9 Several studies have assessed its diagnostic accuracy, often on stored tissue samples; there are discrepancies regarding how to read staining intensity.10 This technique is less expensive and faster, and is therefore proposed as an accurate and affordable first-line method; however, some authors argue that when IHC is negative, molecular testing should be performed.11
The objective of this study was to compare the results of detection of BRAF V600E mutation by IHC and real-time PCR using the cobas®4800 test in a series of patients with a histological diagnosis of PTC. Previous studies had suggested a high agreement rate between the two techniques,12 and validation would confirm IHC (which is more affordable and less time-consuming) as the screening test for the V600E mutation in PTC, similarly to the use of IHC for selecting tissue for the molecular testing of chromaffin tumors.13 A secondary objective was to analyze the clinical and pathological differences between tumors with and without the mutation, which is relevant because of the few publications available on the subject. Our analysis considers the result of evaluation by RT-PCR because this is the reference test for mutation detection.
Patients and methodsA prospective study was conducted in 79 patients diagnosed with papillary thyroid carcinoma between November 2014 and May 2016. The initial tumor tissue samples tested came from the surgical specimen, but from March 2015, tests were performed on samples taken by core needle biopsy, except in cases of incidental carcinomas not diagnosed before surgery. All patients gave their consent to participate in the study, which was authorized by the ethics committee of our hospital.
Immunohistochemistry was performed on tissue fixed for 24h with 10% neutral buffered formalin and using the anti-BRAF V600E (VE-1) mouse monoclonal antibody obtained from a purified cell culture supernatant, at a concentration of 12μg/mL. IHC was done on the Benchmark XT (Ventana Medical Systems) platform, and the specific BRAF protein was viewed with the OptiView DAB IHC Detection Kit (Ventana Medical Systems). Validated PTC specimens with and without the mutation were used as positive and negative controls respectively. The entire process, including antigen unmasking (cell conditioning), incubation with the primary antibody at 37°C, and counterstaining with hematoxylin–eosin before evaluation by the pathologist, took less than six hours (5h and 46min), allowing the report to be issued jointly with the pathology report. The IHC image was interpreted as positive or negative by the pathologist prior to receiving the molecular biologist's report. A sample was considered positive if diffuse cytoplasmic immunostaining was seen; three grades were possible: weak, moderate, and strong, all of which were all reported as positive IHC.
After the area of interest of the tumor, usually the one with the strongest intensity of immunohistochemical marking, had been selected by the pathologist, a fragment from the paraffin block was punched out with a syringe. The sample then underwent deparaffinization (34min) and enzymatic digestion overnight. On the following day, the sample was incubated at 90°C for 1h to inactivate the enzymes and extract DNA, whose subsequent purification required another 85min. After spectrophotometric quantification, a diluted DNA sample (5ng/μL) was analyzed by RT-PCR using the Cobas 4800 BRAF/V600 system specific for that mutation. This process took 90 additional minutes, in which time all the reaction and hybridization cycles took place, allowing for the generation of the automated report. This technique is based on fluorescence resonance energy transfer after a protocol of several cycles of amplification and annealing at various temperatures and durations of BRAF exon 15 (7), which contains codon 600, with the TaqMan® probe. The probe contains an oligonucleotide specific for the V600E mutation, and another in non-mutated (wild type) form, which hybridize with the complementary DNA generated during the PCR cycles. The system performs an automated analysis of the denaturation curve, because PCR products of different length and sequence are denatured at different temperatures depending on the hybridized oligonucleotide, generating peaks that are characteristic for each sequence. The cobas®4800 BRAF/V600 (Roche) software analyzes these peaks and provides a standardized report regarding the presence of the mutation (positive/negative), according to the instructions in the product manual, which is validated by the molecular biologist. It takes almost two complete working days for this process to give the result.
To calculate the costs of each technique, the purchase costs of the reactants and indispensable materials for both tests were used. The time, consumables, or staff required for each were not taken into account because they are considered routine diagnostic tests. For the mutation test with RT-PCR, the cost of 24 diagnostic tests using cobas®4800 BRAF/V600 (Roche) with the TaqMan® probe was €1973.27 (€82.22 each). The cost of 50 anti-BRAF V600E monoclonal antibodies for IHC was €1389, (€27.78 each), and 250 detection kits cost a total of €2785 (€11.14 each). Thus, the total cost of each complete IHC test was €38.92.
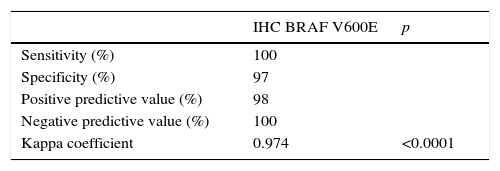
An assessment was made of the clinical and pathological differences between patients and tumors with and without (WT) the BRAF V600E mutation in RT-PCR, considered the reference test. A Student's t test was used to compare the means of the three quantitative variables analyzed, since they all showed a normal distribution (age, tumor size in mm, and preoperative TSH levels). The qualitative variables analyzed included: sex distribution, the presence of extrathyroidal extension, the incidental finding of the tumor, multifocality (more than one tumor focus in the resected thyroid gland), clinical or pathological lymph node involvement (if lymph node dissection was performed), and PTC variant (classic, follicular or mixed). To compare these variables, a Chi-square test was used, or a Fisher's exact test when parameters included fewer than five cases. Sensitivity, specificity, the positive predictive value, and the negative predictive value of IHC were calculated, the mutation-positive tumors by IHC and RT-PCR being considered to be true positives, the WT tumors by both techniques to be true negatives, those with the mutation by IHC but WT by RT-PCR to be false positives, and those not positive by IHC but positive according to the molecular technique to be false negatives. The Kappa coefficient was calculated to assess the agreement of both procedures in mutation detection. SPSS V.20 software (SPSS, Chicago, USA) was used for statistical calculations.
ResultsThe study initially included 84 samples, of which two were discarded, one because of the scarcity of tissue available from which to obtain DNA, and the other due to the poor quality of the histological preparation, which limited IHC interpretation. Overall, 82 samples from 79 patients with PTC aged 12–82 years (mean, 51), 17 of them males, were tested. Two samples were from the same patient, who had two well differentiated tumor sites (one in each lobe), and another two samples were from level IV lateral cervical lymphadenopathies from two patients with thyroid tumor samples also. The mean size of thyroid tumor focus was 14.7mm (range, 4–55mm), with a median of 12mm. Of the 79 glands assessed, 46 contained single-focus tumors, and the remaining 33 (42%) contained between 2 and 8 tumor foci (multifocal), involving both lobes in 23 patients. Eighteen PTCs had an extrathyroidal extension which was microscopic in all cases, and the remaining tumors (61) were limited to the thyroid gland.
Fifty-four analyses were performed on thyroid samples taken from automated 18G core-needle biopsies of the thyroid, as well as from the two adenopathies. Thirteen PTCs, 4–10mm in size, were found incidentally in the pathological examination after thyroidectomy due to size (7), hyperthyroidism (3), patient's decision (2), or biopsy of an adjacent follicular nodule which turned out to be benign after surgery (1). All 13 incidental PTCs were intrathyroidal; one had two foci in the same lobe, and another had four foci distributed in both lobes.
Overall, disagreement was found between the two detection techniques in only one of the 82 techniques, with a negative predictive value of 100% for IHC in our case. Agreement between the two tests was 98.8%, with a disagreement rate of 1.2%. Table 1 shows the statistical values comparing IHC to the reference technique (RT-PCR).
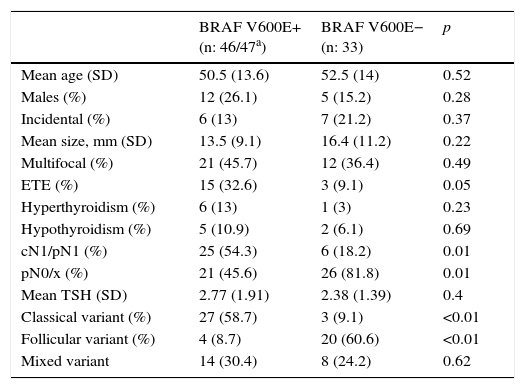
Of the 80 thyroid tissue samples, 47 were positive and 33 were negative by RT-PCR. Table 2 shows the characteristics of the patients according to the results obtained with RT-PCR. Clinical or pathological lymph node involvement and the incidence of the classical variant of PTC were significantly associated with the presence of the BRAF V600E mutation detected by RT-PCR.
Characteristics of the 79 study patients divided by the presence of mutation by RT-PCR.
| BRAF V600E+ (n: 46/47a) | BRAF V600E− (n: 33) | p | |
|---|---|---|---|
| Mean age (SD) | 50.5 (13.6) | 52.5 (14) | 0.52 |
| Males (%) | 12 (26.1) | 5 (15.2) | 0.28 |
| Incidental (%) | 6 (13) | 7 (21.2) | 0.37 |
| Mean size, mm (SD) | 13.5 (9.1) | 16.4 (11.2) | 0.22 |
| Multifocal (%) | 21 (45.7) | 12 (36.4) | 0.49 |
| ETE (%) | 15 (32.6) | 3 (9.1) | 0.05 |
| Hyperthyroidism (%) | 6 (13) | 1 (3) | 0.23 |
| Hypothyroidism (%) | 5 (10.9) | 2 (6.1) | 0.69 |
| cN1/pN1 (%) | 25 (54.3) | 6 (18.2) | 0.01 |
| pN0/x (%) | 21 (45.6) | 26 (81.8) | 0.01 |
| Mean TSH (SD) | 2.77 (1.91) | 2.38 (1.39) | 0.4 |
| Classical variant (%) | 27 (58.7) | 3 (9.1) | <0.01 |
| Follicular variant (%) | 4 (8.7) | 20 (60.6) | <0.01 |
| Mixed variant | 14 (30.4) | 8 (24.2) | 0.62 |
cN/pN: clinical (ultrasound or surgical)/pathological (in microscopic examination) nodal involvement; SD: standard deviation; ETE: extrathyroidal extension; N0: absence of involvement; N1: involvement; pNx: lymph nodes not analyzed.
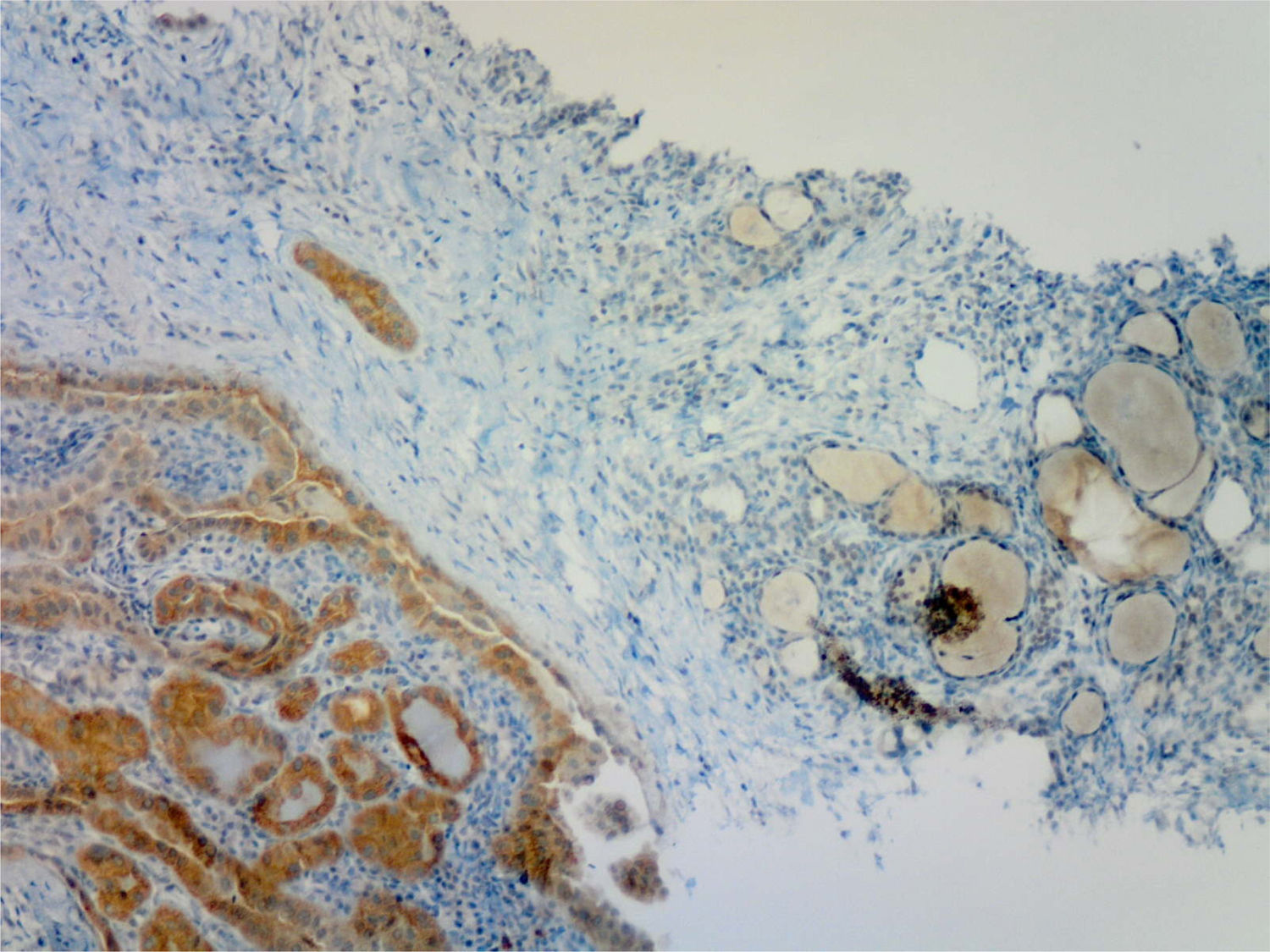
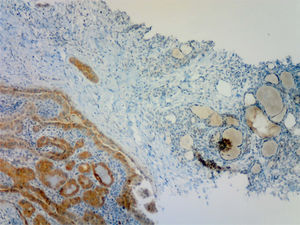
All 47 samples positive by RT-PCR had been previously reported by the pathologist as positive based on IHC (Fig. 1). Of the 33 thyroid samples without BRAF V600E mutation by RT-PCR, one was reported as (moderately) positive by IHC. This sample corresponded to a 5mm incidental papillary carcinoma, predominantly of the follicular variant, non-encapsulated, with discrete areas of classical pattern, unifocal and intrathyroidal, adjacent to a follicular adenoma, in a 38-year-old woman. This positive immunostaining was reviewed and confirmed by the pathologist, who also confirmed that the sample for genetic testing had been adequately taken from the area of malignant tissue with a stronger signal intensity in IHC.
The two metastatic lymph nodes analyzed were positive by both RT-PCR and IHC. Paradoxically, in one of the patients the primary thyroid tumor was negative by both techniques. In the patient with two independent PTC sites, one in each lobe, both were positive by the two techniques.
Financial expenditure, limited to the cost of test-specific materials, for the 82 RT-PCR tests during the 18 months of the study was €6740, as compared to €3182 for the IHC materials. The IHC results were received at the same time as the pathology report, whereas the RT-PCR molecular analysis was received one or more days after the pathology report.
DiscussionThe BRAF V600E mutation does not occur in benign thyroid lesions, and has therefore been proposed as the best method for samples with indeterminate cytology obtained by fine needle aspiration.14 The presence of the mutation leads to constitutive activation of the pathway signaled by MAP (mitogen-activated protein) kinases, which results in increased cell proliferation and resistance to apoptosis. It has been shown to be associated with predictors of tumor aggressiveness in PTC, mainly including extrathyroidal extension and lymph node involvement, and to an increased risk of recurrence, but not of mortality.3 Moreover, the mutation decreases tumor sensitivity to radioiodine through the translocation of the NIS (Na/I symporter) from the membrane to the cytoplasm, preventing entry of the isotope into the cancer cell.15 The mutation has also been shown to be a potential therapeutic target for BRAF inhibitor drugs,16 similarly to vemurafenib treatments for malignant melanoma. This diagnostic and therapeutic relevance makes its detection (ideally before surgery) very useful, but the complexity and economic cost of molecular techniques, and the need for a molecular biology laboratory, are limiting factors for many hospitals where this disease is managed.
The frequency of the mutation in Spain has been reported in two publications,5,15 and is around 50%. In our sample of 79 patients, 46 (58.2%) were found to have the mutation using RT-PCR, which represents a slightly higher frequency. Analysis of the characteristics of these patients also confirms a significant association in Spain with the presence of lymph node metastases and the classical variant of papillary carcinoma. In addition, in agreement with reports from other countries, it tends to be detected in tumors that are smaller and have extrathyroidal extension, which supports its higher biological aggressiveness.
IHC has been validated as a helpful marker of the presence of mutations in the succinate dehydrogenase (SDH) gene in chromaffin tumors, so that molecular evaluation for germline mutations in any of the various SDH genes is indicated only in patients with tumors negative to IHC marking with SHDB. Conversely, its expression and positivity by IHC obviates the need to assess these genes, as they are incompatible with mutations.17 In addition, a stain negative for SDHA allows for the targeting of this specific gene in the test.
The development of a mouse monoclonal antibody (VE-1) has permitted the evaluation of IHC for the BRAF V600E mutation in PTCs and other tumors with mutation in this allele, mainly melanoma and colorectal carcinoma.18 Several recent publications have found a high correlation between IHC and DNA analysis by various molecular techniques.12,18–20 IHC has limitations derived from pre-analytical factors because, as occurred in one of our cases, sample quality may sometimes not allow for a definitive verdict. To limit this, it is recommended that tissues be fixed within two hours of collection, and processed carefully.18 Another limitation stems from the subjectivity of interpretation, and many publications distinguish degrees of positivity. As this study was prospective and performed before DNA analysis, a single dichotomous category (positive/negative), judged by the pathologist, was used for IHC. It should be noted that testing may be performed on samples taken from a core-needle biopsy, as previously reported by others.21,22 This technique allows larger samples of a better quality to be obtained as compared to cytological aspiration, in which immunocytochemical labeling for BRAF V600E has also been used.23
Among the studies using various molecular analysis techniques published in the past three years,10,11,19 special mention should be made of a meta-analysis24 of 11 studies (with cytology samples in two) comprising 1141 patients and using various DNA analytical techniques. This meta-analysis found a high agreement rate between IHC and DNA evaluations, especially when IHC was positive (92.1%). There was more disagreement in samples negative by IHC (IHC false negatives), which was explained by the use of old, low-quality histological samples which had been previously frozen or decalcified. There were no false negative IHC results in the present study, as the only sample of questionable quality was discarded, and all the tissues tested had been fixed a few hours previously. Other possible causes of IHC false negatives are the loss of expression of the mutated antigen, described in necrotic tumor areas,25 and the presence of additional mutations that prevent the translocation of the mutated messenger RNA into a functional protein.
The results of this study agree with those reported by Zhu et al. using Sanger sequencing26 in terms of an IHC sensitivity of 100%, although they found a lower specificity (82.2%). This lower specificity may possibly be due to the fact that the study used samples fixed years before and to the use of Sanger sequencing, which is less sensitive to low percentages (<10%) of mutation-positive tumor cell populations. IHC false positives, the other potential source of disagreement between IHC and other molecular techniques, have been attributed to sample contamination with other tissues (sinus mucosa or bronchioles) with positive immunoreactivity27 (ruled out in our study), or to a false negative RT-PCR. This possibility is less likely than with Sanger sequencing because the test detects mutated cells at percentages lower than 5%, but the presence of mutation in a small proportion of tumor cells due to genetic heterogeneity of the tumor appears to be the most plausible explanation. The discordant tumor was a PTC with a predominance of the follicular variant, usually lacking a BRAF mutation, which suggests the presence within the tumor of a mutation-positive cell clone not included in the sample taken for the molecular test, but responsible for IHC positivity. The cellular and genetic heterogeneity of PTC is reflected in the patient with two discordant tumor sites in mutational analysis: an intrathyroidal unifocal PTC of the follicular variant that was BRAF-negative by both techniques, and a nodal metastasis of this tumor at level IV cervical lymph nodes whose mutation was evident by both techniques. This combination suggests early dissemination of a cell clone with the V600E mutation, an early event in PTC carcinogenesis,28 which was not identified in the primary tumor by either technique, presumably due to low cell density. In these cases, the use of even more sensitive molecular techniques such as peptide nucleic-acid-clamp quantitative PCR (PNA-clamp qPCR) may improve sensitivity in detecting the mutated population.11
The absence of false negative IHC results when tissue samples are of good quality, such as in our series, reaffirms the possibility of using IHC as a screening technique, which, in turn, implies significant cost savings. In addition, this procedure requires a significantly shorter time than molecular biology testing. Fewer human resources are also needed to perform IHC, as the entire test is done in the same department and on the same sample already processed for the standard histological examination, whereas sequencing requires the participation of the pathology department to obtain the tumor sample, and of the molecular biologist to perform genetic testing.
An obvious limitation of this study is the number of samples tested, only 82, all of them of excellent quality, but insufficient in number to allow definitive conclusions to be drawn. Assessment with a larger, ideally multicenter, study could validate the results obtained in this study.
To sum up, IHC for BRAF V600E, compared to RT-PCR, appears to be an accurate technique for identification of the BRAF V600E mutation in PTC, and is associated with substantial cost and labor savings.
Conflicts of interestAll the authors explicitly state that they have no conflicts of interest regarding the contents of this article or the techniques employed.
We thank the technicians of the pathology department for their help with specimen preparation.
Please cite this article as: Paja Fano M, Ugalde Olano A, Fuertes Thomas E, Oleaga Alday A. Detección inmunohistoquímica de la mutación BRAF V600E en el carcinoma papilar de tiroides. Evaluación frente a la reacción en cadena de la polimerasa en tiempo real. Endocrinol Diabetes Nutr. 2017;64:75–81.