Ectopic thyroid is a rare entity defined as any thyroid tissue not located in its usual position anterior to the laryngeal cartilages. Most frequent ectopic thyroid is due to the absence of regression from the thyroglossal duct. However, others ectopic thyroid tissues can be found during differential diagnosis of neck nodules.1 Parasitic thyroid nodules, also called sequestered, detached or accessory thyroid nodule are separated thyroid nodules from the main thyroid gland located in the lateral neck area.2 Therefore, differential diagnosis of thyroid tissue outside the thyroid gland may involve a complex process.3 Here, we present a patient with parasitic thyroid nodule.
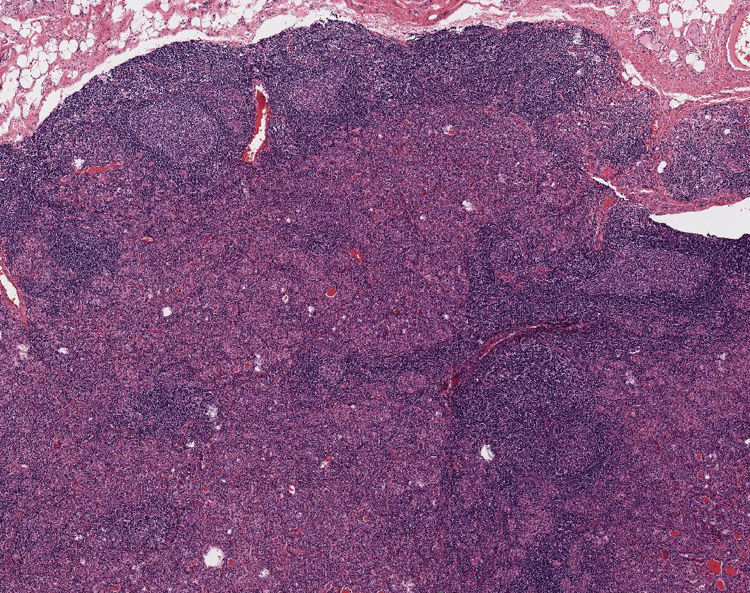
Case presentationA 49-year-old woman was referred to our department in 2016 due to a goitre detected during a dysphagia differential diagnosis. Cervical ultrasonography discovered a multinodular goitre with a predominant hypervascularised 30mm isthmic thyroid nodule. Subsequent fine needle aspiration biopsy (FNAB) showed follicular proliferation. Finally, she underwent a total thyroidectomy with histopathological results of lymphocytic thyroiditis and nodular hyperplasia. A single 3mm papillary thyroid microcarcinoma was discovered incidentally in the right thyroid lobule. During the follow-up, a clinically thyroglobulin (Tg) rise was detected (supressed Tg from 4.6 to 18ng/mL) with persistently high anti-Tg antibodies (levels close to 300U/mL). Consequently, Tg was measured following TSHhr stimulation showing a rising to 43ng/mL. Cervical ultrasonography showed three concurring suspicious bilateral level-IV lymphadenopathies. Patient did not undergo a lymph node FNAB washout for Tg because it was not yet a widely established technique in our hospital. Thus, extended bilateral lymphadenectomy was performed one year later. Histopathology demonstrated no features of malignancy. In the neck dissection, there were sixteen normal lymph nodes and two bilateral nodules (12mm and 15mm) of hyperplastic thyroid tissue not related to lymph nodes. These nodules showed benign histological features with lymphocytic thyroiditis (Fig. 1). There were no nuclear features of papillary thyroid carcinoma. Galectin 3, CK19 and HBME1 immunohistochemistry was negative. Hence, the nodules were classified as benign parasitic nodules.
DiscussionOur case entailed a complex differential diagnosis of thyroid tissue outside the thyroid gland, finally due to parasitic nodules. The parasitic thyroid nodule occurs when thyroid tissue located in the lateral neck has no relationship or association with the lymph nodes, and may be defined as a thyroid nodule entirely separate from the thyroid or attached to it by a narrow pedicle, presenting the same histology and in the same facial plane as the thyroid, and should not be associated with lymph nodes.3 About one hundred cases of parasitic thyroid nodules have been described, most of them are single subcentimetric isolated nodules.4 Patient parasitic thyroid nodules were not associated with lymph nodes or the thyroid gland. Similar histology (lymphocytic thyroiditis) to main thyroid tissue was also confirmed.
The differential diagnosis of the case with two parasitic thyroid nodules proved to be complex and next possible diseases were considered: thyroid rests from embryological origin, metastatic thyroid cancer lymphadenopathies, seeding from prior thyroid surgery and thyroid tissue clefting. Firstly, thyroid rests from embryological origin are often located in the neck midline, whereas our suspicious images were detected in cervical level IV, laterally to the theoretical line of the thyrothymic tract.5 Secondly, metastatic thyroid cancer was considered a potential differential diagnosis due to patient previous medical history. When prominent lymphocytic infiltration is observed in a parasitic nodule pathologist must be aware of this rare condition and differentiate from a condition more frequent, a metastatic carcinoma in a lymph node. On occasion this can be very difficult and immunohistochemical techniques can be necessary to solve the problem. In our case, no association of the hyperplastic nodules with lymph node tissue or nuclear features of papillary thyroid carcinoma were observed. Both parasitic thyroid nodules of the same patient showed benign histopathological features and immunohistochemistry for Galactin 3, CK19 and HBME1 were negative.6 Unfortunately, BRAF and other thyroid cancer gen-mutation studies were not available in our centre at that moment, these techniques would have helped in the patient's clinical management. Another differential diagnosis included thyroid seeding from previous surgery. “Thyroidosis” usually happens in the subcutaneous tissue or in the field of a previous surgery.7 However, in our case the bilateral neck thyroid nodules were not within the operative field of the original total thyroidectomy which did not include any neck lateral adenopathy dissection. Finally, thyroid tissue scission is a well-known phenomenon accompanying lymphocytic thyroiditis in patients with multinodular goitre. In fact, tissue clefting was found in a recent detailed description of a parasitic thyroid nodule series. It is proposed that the mechanical action of the neck muscles is the main mechanism of these parasitic thyroid nodule's origin, which may separate a portion of the goitre through the muscular fascia.3 Therefore, our diagnosis considering all the clinical and pathological features, was of two parasitic thyroid nodules probably due to tissue clefting, although thyroidosis origin could not be utterly rejected.
We concluded that parasitic thyroid nodules should be included in the differential diagnosis of lateral neck masses. This rare entity may be the cause of a serious clinical dilemma if an important medical decision is to be made due to a malignant thyroid suspicion.
Patient consentPatient signed the informed consent.
FundingNo funding was received for this work
Conflict of interestThe authors wish to confirm that there are no known conflicts of interest.