The optimal initial dose of subcutaneous (SC) insulin after intravenous (IV) infusion is controversial, especially in patients receiving continuous enteral nutrition (EN) or total parenteral nutrition (TPN). The aim of this study was to evaluate the strategy used at our hospital intensive care unit (ICU) in patients switched from IV insulin to SC insulin glargine while receiving EN or TPN.
Design and methodsA retrospective analysis was made of 27 patients on EN and 14 on TPN switched from IV infusion insulin to SC insulin. The initial dose of SC insulin was estimated as 50% of the daily IV insulin requirements, extrapolated from the previous 12h. A corrective dose of short-acting insulin (lispro) was used when necessary.
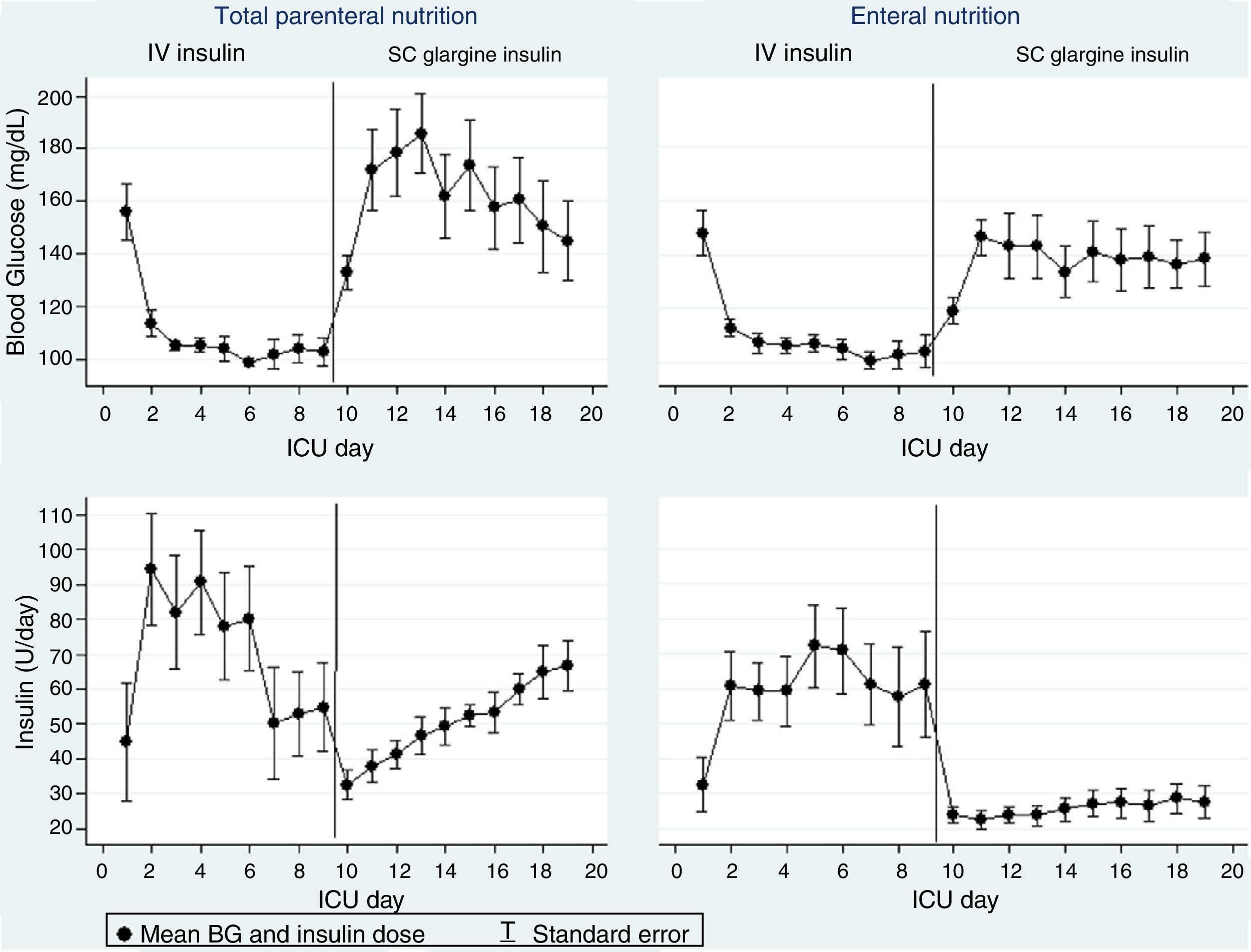
ResultsMean blood glucose (BG) level during SC insulin treatment was 136±35mg/dL in the EN group and 157±37mg/dL in the TPN group (p=0.01). In the TPN group, mean BG was >180mg/dL during the first three days after switching, and a 41% increase in the glargine dose was required to achieve the target BG. In the EN group, mean BG remained <180mg/dL throughout the days of transition and the dose of glargine remained unchanged.
ConclusionsIn the transition from IV to SC insulin therapy, initial insulin glargine dose estimated as 50% of daily IV insulin requirements is adequate for patients on EN, but inadequate in those given TPN.
La dosis óptima inicial de insulina subcutánea (SC) después de la infusión intravenosa (IV) es controvertida, especialmente en pacientes que reciben nutrición enteral continua (NE) o nutrición parenteral total (NPT). El objetivo de este estudio fue evaluar la estrategia utilizada en nuestra unidad de cuidados intensivos (UCI) en pacientes sometidos a transición de infusión IV a insulina glargina SC mientras recibían NE o NPT.
Diseño y métodosSe analizaron retrospectivamente 27 pacientes con NE y 14 con NPT que cambiaron de infusión IV a insulina SC. La dosis inicial de insulina SC se estimó como el 50% de los requerimientos diarios de insulina IV, extrapolado de las 12 horas anteriores. Se utilizó dosis correctiva de insulina ultrarrápida (lispro), cuando fue necesaria.
ResultadosLa media de glucemia plasmática (GP) con insulina SC fue de 136,35mg/dl en el grupo NE y de 157,37mg/dl en el grupo NPT, p=0.01. En el grupo de NPT la GP media fue>180mg/dL durante los tres primeros días después de la transición y fue necesario un aumento del 41% en la dosis de glargina para alcanzar la GP objetivo. En el grupo NE, la GP media permaneció<180mg/dl durante los días de transición y la dosis de glargina permaneció sin cambios.
ConclusionesEn la transición de la terapia de insulina IV a insulina SC, la dosis inicial de insulina glargina estimada como el 50% de los requerimientos diarios de insulina IV es adecuada para los pacientes que reciben NE, pero insuficiente para los que reciben NPT.
Hyperglycemia is associated with an increase death and infection among patients hospitalized in ICU.1,2 Insulin is the preferred agent for glycemic control in hospitalized patients and, in the ICU setting, insulin is usually administered as IV continuous infusion, which is the most effective and safe method for achieving the glycemic targets.3,4 When a patient's condition improves many guidelines and recommendations suggest switching from IV insulin to SC insulin.5–8 The decision to transfer the patient from IV to SC insulin should be made carefully, evaluating the patient clinical situation, recognizing factors that influence a safe transition and calculation of proper SC insulin doses.9
Outside ICU setting, use of basal-prandial-correction therapy with SC insulin analogs constitutes the preferred regimen5–7,10 with careful monitoring of BG to achieve the target range and avoid hypoglycemia. In patients receiving continuous EN or TPN, basal-correction therapy constitutes a suitable method for managing hyperglycemia6,11,12 and the dose of insulin is normally higher for maintained target BG.
Few studies have focused on the optimal transition from IV insulin infusion to SC insulin therapy13–16 and the optimal dose of initial SC insulin is highly uncertain, particularly in patients in whom artificial nutrients are delivered continuously.12,17
This study attempts to evaluate the strategy used in hospitalized patients in ICU undergoing transition from IV insulin infusion to SC long-acting insulin glargine while receiving EN or TPN, and has tried to determine the optimal dose of insulin needed to maintain glycemic goals.
Material and methodsWe established a program of intensive insulin management for hyperglycemia, designed to achieve a glycemic target of 100–140mg/dL in a 16-bed medical-surgical ICU. BG was measured on admission to the ICU, and IV insulin was begun for all patients whose glucose levels were greater than 140mg/dL. During a period of 6 months, 120 patients requiring a continuous insulin infusion were included in this retrospective study. Transition to SC insulin occurred in 74 patients requiring >1IU/h IV insulin when critical illness was resolved and nutritional status was stable. Patients receiving EN (n=27) or TPN (n=14) and treated with basal insulin glargine plus correction insulin lispro were eligible for inclusion in the study.
The insulin drip rate in the preceding 12h was used to calculate initial SC insulin dose. The average rate was multiplied by 24 to calculate the total daily insulin requirements, and 50% was administered SC as the first daily injection of glargine. On the basis of capillary BG monitoring (Accu-Chek Sensor, Roche, Mannheim, Germany) every 6h, correction doses of insulin lispro were administered if glycemia remained above 140mg/dL. Glargine insulin dose was increased or decreased by 10–20% every day to achieve a glycemic goal of 100–140mg/dL. Mean BG levels and mean insulin dose were evaluated during IV and SC insulin treatment.
The study was approved by the Ethics Committee of our institution.
Data are given as mean±SD. Group differences were analyzed by χ2 test for categorical variables and t-test for continuous variables. The SPPS V 20.0 was used to analyze the data. Statistical significance was set at two-sided p value<0.05.
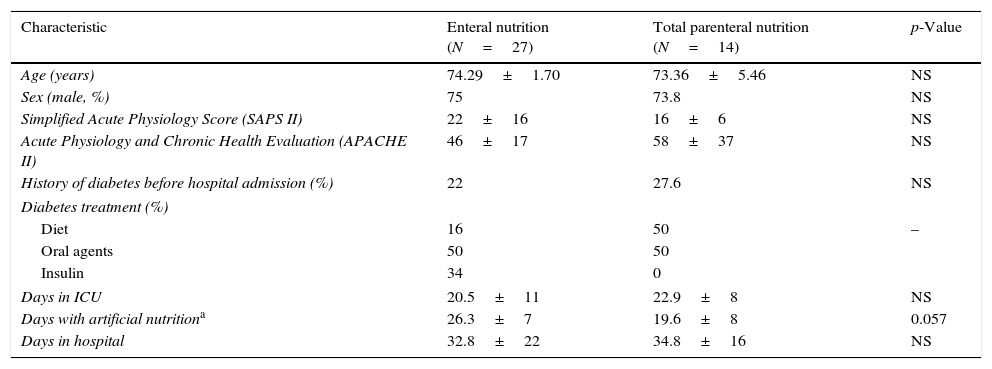
ResultsThe baseline characteristics of patients with EN and TPN are displayed in Table 1.
Main baseline characteristics of the population (N=41).
| Characteristic | Enteral nutrition (N=27) | Total parenteral nutrition (N=14) | p-Value |
|---|---|---|---|
| Age (years) | 74.29±1.70 | 73.36±5.46 | NS |
| Sex (male, %) | 75 | 73.8 | NS |
| Simplified Acute Physiology Score (SAPS II) | 22±16 | 16±6 | NS |
| Acute Physiology and Chronic Health Evaluation (APACHE II) | 46±17 | 58±37 | NS |
| History of diabetes before hospital admission (%) | 22 | 27.6 | NS |
| Diabetes treatment (%) | |||
| Diet | 16 | 50 | – |
| Oral agents | 50 | 50 | |
| Insulin | 34 | 0 | |
| Days in ICU | 20.5±11 | 22.9±8 | NS |
| Days with artificial nutritiona | 26.3±7 | 19.6±8 | 0.057 |
| Days in hospital | 32.8±22 | 34.8±16 | NS |
Data are mean±standard deviation.
There were no significant differences between EN and PN groups in sex distribution, mean age, percentage of patients with previous diabetes (22.2% vs. 28.6%), severity of illness at ICU admission defined by APACHE II (22±16 vs. 16±6) or SAPS II (46±17 vs. 58±37) scores. The distribution of the most common admission diagnoses was also similar in both groups.
Mean BG during IV insulin treatment (113±2 vs. 112±2mg/dL) was similar between groups. The average insulin drip rate in the 12h prior to conversion to SC insulin was 2.0U/h in the EN group and 2.8U/h in the TPN group.
The initial glargine dose (23±12 vs. 33±15IU, p<0.05) was significantly higher in patients with TPN.
During SC insulin treatment, the mean BG was 136±35mg/dL vs. 157±37mg/dL in EN and TPN, respectively, p=0.01. TPN was associated with a transient higher mean glucose in the first 3 days after the switch to glargine (Fig. 1), being>180mg/dL in 35–50% of patients, whereas, the mean BG remained <180mg/dL in the EN group. In patients receiving TPN, glargine dose needed to be increased by 41%, whereas in EN group in the transition period the dose of glargine was increased only about 5% (Fig. 1). Two episodes of blood glucose<40mg/dL occurred in the EN group due to protocol violations.
DiscussionIn the present study we observed that basal-correction insulin therapy with insulin analogs was effective and safe in ICU patients receiving continuous EN or TPN. Estimating the initial glargine dose as 50% of the daily insulin requirements, extrapolated from the average IV insulin infused in the previous 12h, was suitable for patients with continues EN, but insufficient for patients with TPN.
Use of basal-prandial-correction therapy with insulin analogs constitutes a suitable regimen for inpatient management of hyperglycemia.10,18 Patients receiving continue EN or TPN require basal insulin therapy plus a correction dose every 4–6h, but they do not need prandial doses since nutrients are delivered continuously. Our study confirms and extends reports that support the use of long-acting insulin glargine in patients with EN or TPN.12,17,19
Switching from continuous IV insulin infusion to SC therapy is a complex matter that requires evaluation of the patient's condition, nutritional treatment and recent insulin dosage. In the present study, transition to a SC insulin regimen was undertaken once the critical illness had resolved, all patients received EN or TPN and insulin requirements were stable.
To avoid rebound hyperglycemia after transition from IV to SC insulin require adequate estimation of subcutaneous insulin dose, and sufficient duration of overlap of the insulin infusion with the subcutaneous insulin.13 Planned transition requires that the first dose of SC insulin is administered at least 1h for short-acting SC insulin and ideally 3–4h for long-acting SC insulin, prior to discontinuation of the infusion. Initial SC insulin requirements are usually extrapolated from the average infusion rate measured during a stable period of continuous IV insulin infusion and several dose algorithms has been proposed. However, there are no conclusive data about the optimal conversion factor to calculate the initial subcutaneous insulin dose.13,14,16,20 Our findings show that conversion to glargine at a dose based on the previous 12-h insulin requirements maintains the average BG targets, without need to increase glargine insulin dose in patients with EN. Nevertheless, in patients with TPN, a significant increase in glargine was needed, particularly in the first three days after the switch.
The higher dose of insulin needed in the TPN group can be explained by the higher hyperglycemic potential of TPN.21–23 Although data are scarce regarding the influence of the feeding route on insulin secretion, the higher insulin requirements with TPN could be due, at least in part, to the lack of stimulation of the incretin system that determines lower beta-cell response to intravenous glucose and facilitates glucagon release.22,24–26 In critically ill patients, hyperglycemia it may also reflect the increase in insulin resistance.23,27
Limitations of the present study are related to retrospective design, sample size, the inclusion of patients with and without DM, and that it was conducted at a single tertiary care center. However, it reflects the application of a management protocol for hyperglycemia in the usual clinical practice, patients are likely to be representative of the admitted to a medical-surgical ICU and our findings could help fill the gap in the literature on the transition from IV to SC insulin in patients with continuous artificial nutrition. Nevertheless, it would be helpful to conduct similar studies comparing different protocols at other institutions to obtain results that have greater generalizability.
On the basis of our findings, for patients receiving EN we recommend an initial SC insulin glargine dose equal to 50% of the overall daily insulin requirements extrapolated from the infusion rate 12h before transition. Future studies could be conducted to determine the optimal dose of initial SC insulin in PN.
FundingThis research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interestAPP has participated as a consultant for, or has received lecture fees or travel reimbursement from Sanofi-Aventis, Esteve, GSK, Almirall, Novo Nordisk, Eli Lilly, MSD, Boehringer Ingelheim, Novartis, Menarini, Janssen and Astra Zeneca. None of the other authors have any conflict of interest.