Hypoglycemia is defined as plasma glucose levels less than 50mg/dL.1 However, the definition of hypoglycemia may vary depending on whether glucose is measured in venous or capillary blood. The cut-off point of the glucose level that triggers a physiological response to hypoglycemia and the resultant symptoms may also vary. The diagnosis of hyperglycemia is based on Whipple's triad: low blood glucose level, symptoms of hypoglycemia, and symptom improvement once blood glucose returns to normal. Hypoglycemia causes adrenergic symptoms such as tachycardia, palpitations, tremor, sweating, pallor and anxiety, and non-adrenergic or neuroglycopenic symptoms including hunger, headache, weakness, visual disturbances, confusion, lethargy, seizures, and even coma.1 The most common cause in our environment is glucose lowering treatment (oral hypoglycemic drugs and insulin). Other potential causes include end-stage renal failure, sepsis, hormone deficiencies, big mesenchymal tumors, insulinoma, congenital metabolic diseases, etc. Plasma glucose levels in the hypoglycemic range not associated with clinical signs are sometimes detected. Such cases may be due to the inadequate perception of hypoglycemic symptoms or to “factitious hypoglycemia”. The case of a patient with an uncommon cause of hypoglycemia is reported below.
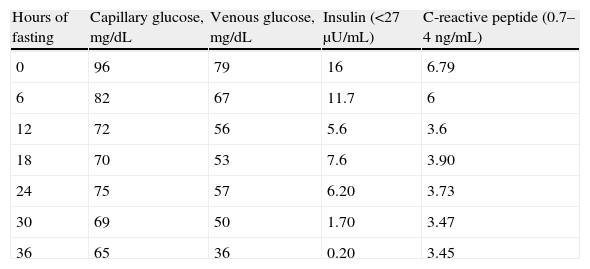
The patient was an 83-year-old male with a history of high blood pressure treated with spironolactone 25mg daily and furosemide 20mg daily, hypercholesterolemia treated with atorvastatin 40mg daily, chronic renal failure secondary to nephroangiosclerosis, paroxysmal atrial fibrillation, cognitive impairment, vascular parkinsonism, and depressive syndrome. He had also been seen by different specialists due to frequent presyncopal episodes and based on laboratory tests reporting a WBC count of 22,400/μL, a RBC count of 6.3×106/μL, hemoglobin 17.1g/dL, hematocrit 53%, and a platelet count of 441,000/μL, he had been diagnosed with moderate pancytopenia. Tests for myeloproliferative syndrome were negative. The patient was referred to endocrinology to rule out hypoglycemia as the cause of syncopal episodes after a venous fasting blood glucose level of 41mg/dL was found. No history of diabetes or use of hypoglycemic drugs was found. Presyncopal episodes were not related to intake or fasting. The physical examination was normal. Prior laboratory tests found blood glucose levels ranging from 40 to 70mg/dL. At this first visit, he was given a glucometer to record capillary blood glucose in the event of presyncopal symptoms and instructions to follow a fractionated diet. At subsequent visits, the patient provided capillary blood glucose measurements revealing no hypoglycemia. Laboratory tests results included: creatinine 1.6mg/dL, glomerular filtration rate 44mL/min/1.73m,2 lactate dehydrogenase 1440IU/L (313–618), chromogranin A 769ng/mL (19–98), C peptide 6.81ng/mL (0.7–4), and insulin 10.7μgIU/mL (<25μU/mL), with 41mg/dL blood glucose. Results of additional tests were: C peptide 3.64ng/mL and insulin 16.5μgIU/mL, with a blood glucose level of 56mg/dL. C-reactive peptide levels were interpreted taking into account that its clearance was decreased due to the presence of renal failure. Chromogranin A, used as a biochemical marker of neuroendocrine tumors, may be falsely elevated in patients with renal failure and poorly controlled high blood pressure. Abdominal ultrasound revealed no remarkable findings in the pancreatic head and body, and a homogeneous 12.5cm spleen. Computed tomography (CT) of the abdomen and pelvis, which was performed without contrast due to renal failure, showed a non-specific nodular lesion some millimeters in size in the pancreatic tail, homogeneous splenomegaly, and lytic lesion at the T11 vertebra. Because of the low blood glucose levels found in several measurements, with detectable insulin and C-reactive peptide, together with the occurrence of a pancreatic lesion, admission for hypoglycemia work-up was decided. A fasting test was discontinued at 36h due to a venous blood glucose level of 36mg/dL, with no symptoms of hypoglycemia and decreased insulin levels (Table 1).
The main pathophysiological characteristic of endogenous hyperinsulinism is lack of suppression of insulin secretion during hypoglycemia. This causes the occurrence during the fasting test of plasma insulin levels higher than 6μU/mL and C-reactive peptide levels higher than 0.6ng/mL with plasma glucose levels less than 45mg/dL and symptoms of hypoglycemia. The results of that test therefore ruled out the existence of endogenous hyperinsulinism and demonstrated a marked discrepancy between capillary and venous blood glucose levels. Such variability depends on factors such as the glucometer used and the adequate performance of the procedure.
Because of the elevated WBC count, lack of hypoglycemic symptoms, and disagreement between glucose levels measured by the glucometer and in venous blood, it was decided to rule out glucose consumption by blood cells. For this, two samples were taken, of which one was centrifuged immediately and the other after 50min, the estimated usual time for sample arrival at the laboratory. Levels found in these two samples were 61mg/dL and 46mg/dL, respectively. These results suggested decreased plasma glucose levels due to glucose consumption by blood cells and ruled out the existence of true hypoglycemia. The patient refused to undergo additional tests to further characterize the pancreatic nodule and lytic lesion found in abdominal CT.
Hypoglycemia due to glucose consumption in blood cells is usually not easy to recognize, unlike as occurs with hypoglycorrhachia in bacterial meningitis.2 Glucose circulating in blood is usually consumed by RBCs and WBCs by glycolysis, a process that converts glucose into pyruvate.3 In the presence of a myeloproliferative syndrome, leukemic states, or leukocytosis from other causes, glucose consumption by blood cells increases, and low glucose levels may be found.1,4–6,8In vitro decrease in glucose is directly related to sample incubation time and temperature and to WBC count.2 At room temperature, glucose levels decrease from 7 to 20mg/dL/hour, regardless of the initial glucose level.7 High temperatures also accelerate the glycolytic process.3 The rate of glycolysis is also higher in blood from patients with leukocytosis. However, this phenomenon is independent of WBC type (lymphocytes or polymorphonuclear), and the “blood glucose/leukocytosis” ratio is specific for each individual.7 On the other hand, the glycolytic process was once thought to be faster when leukocytosis was at the expense of immature forms. However, when blood samples from patients with leukemia were compared to blood samples from healthy individuals with similar WBC counts, glucose consumption by leukemic cells was shown to be less than one-fifth of the consumption by non-leukemic cells, except when the WBC count exceeded 60,000/μL. Thus, the in vitro glucose consumption rate is related to WBC count rather than to cell maturation or differentiation.9
There are several ways to prevent glucose consumption in vitro.10 First, high temperatures should be avoided, as well as delay in sample testing. It is recommended that the sample be kept at a temperature of approximately 4°C and that transportation time be minimized. Time periods longer than two hours from sampling to testing have been associated with significant drops in blood glucose.11 Second, early centrifugation of the sample is recommended. Finally, to avoid this phenomenon, blood samples should be drawn into tubes containing inhibitors of glycolysis such as sodium fluoride or potassium oxalate. However, as these compounds may interfere with some laboratory methods, their use now tends to be avoided.
Hypoglycemia due to blood cell consumption should be suspected in the event of marked leukocytosis for any reason, venous hypoglycemia with no symptoms of hypoglycemia and no improvement after glucose administration (Whipple's triad), or a potential mistake in sample collection or processing.
Multiple classifications of the causes of hypoglycemia are available, but few of them mention the cause reported in this case. It is advisable to be aware of and to suspect this phenomenon so as to prevent false diagnoses and unnecessary tests.
Please, cite this article as: Gutiérrez Medina S, et al. Hipoglucemia facticia. Endocrinol Nutr. 2013;60:147–9.