Association of hyperthyroidism and pregnancy is not an unusual event, and has an impact on both the mother and fetus. After delivery, it may also affect the newborn and the nursing mother. Clinical management of this situation is quite different from that required by non-pregnant hyperthyroid women and poses significant diagnostic and therapeutic challenges.
This review addresses aspects related to the unique characteristics of biochemical assessment of thyroid function in pregnancy, the potential causes of hyperthyroidism in pregnancy, and the clinical and therapeutic approach in each case. Special attention is paid to pregnancy complicated with Graves’ disease and its different the maternal, fetal, neonatal, and postnatal consequences.
La coexistencia de hipertiroidismo con la gestación no es un fenómeno excepcional. Cuando esto curre la repercusión es tanto materna como fetal e incluso, tras el parto, puede afectar al neonato y a la puérpera. El manejo clínico de esta situación es radicalmente distinto al del hipertiroidismo de la no gestante y plantea importantes retos tanto desde el punto de vista diagnóstico como terapéutico.
En esta revisión se examinan los aspectos relacionados con las peculiaridades de la valoración bioquímica de la función tiroidea en el embarazo, las diferentes posibilidades etiológicas ante la aparición de un hipertiroidismo en la gestación y el enfoque clínico terapéutico en cada caso. Se dedica especial atención a la gestación complicada con una enfermedad de Graves diferenciando su repercusión a distintos niveles: materno, fetal, neonatal y puerperal.
The prevalence of thyroid function changes in women is very high (5/1000 for hypothyroidism and 3/1000 for hyperthyroidism1), and since many of these conditions occur at a childbearing age, there is nothing strange in the idea of pregnancy being associated with these diseases. In addition, as the clinical practice of measuring thyroid hormone (TH) levels in pregnant women is extended, an increasing number of thyroid function abnormalities which would have been overlooked in other circumstances are now being detected in pregnant women.
On the other hand, maternal thyroid function undergoes significant changes during pregnancy resulting in changes in the different laboratory parameters. Because of this, a different approach should be used in pregnant as compared to non-pregnant women when interpreting the different thyroid function parameters in order to avoid wrong diagnoses and assessments with potentially severe consequences.
As a consequence of the foregoing, interest has arisen in recent years in the physiology and the potential changes of the thyroid gland during pregnancy.2 Among these, we have reviewed aspects related to hyperthyroidism in pregnancy, its challenges, and its potential consequences, both during pregnancy (in the mother and/or fetus) and after birth (in the mother and/or newborn).
Functional thyroid assessment during pregnancySignificant physiological changes which have a considerable impact on the different parameters of maternal thyroid function3,4 occur during pregnancy, including the following:
- -
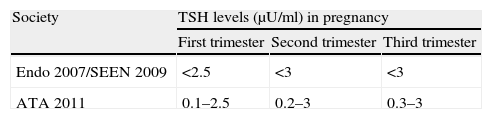
TSH. Decreased TSH levels have traditionally been seen in any pregnancy period as compared to the levels found in non-pregnant women,5 with minimum levels in the first trimester which subsequently increase in the second and third trimesters. These changes in TSH levels mirror the changes in human chorionic gonadotropin (HCG) levels, which peak in the first trimester and gradually decrease thereafter. This is why the different scientific societies have established normal reference values6–8 (Table 1).
It should be noted that decreases in TSH levels are even greater in multiple pregnancies, and that pregnant women who smoke have lower TSH levels during the first and third trimesters.9
- -
TBGT-T4. Increased estrogen levels associated with pregnancy increase TBG levels and, consequently, TT4 values. However, changes in TT4 levels are very consistent (they increase by 150%) and do so independently of both the trimester and the method used. Thus, if TT4 levels are available, T4 production by pregnant women may be estimated by multiplying by 1.5 the reference limits in non-pregnant women.
- -
FT4. In clinical practice, most free T4 (FT4) measurements are not performed using the gold standard equilibrium dialysis or ultrafiltration methods. The procedure routinely used is an immunoassay system where FT4 is not a directly quantitated value, but rather estimated through a protein-sensitive method (influenced by TBG and albumin), and which is therefore strongly modified by pregnancy, and also varies depending on the trimester. In addition, each trial method has specific variations. This means that, for an adequate interpretation of FT4 during pregnancy, reference values depending on the gestational age and method used must be available, as stated by the American Thyroid Association (ATA).6
As shown in Table 2, hyperthyroidism during pregnancy may have serious consequences for both the mother and fetus and, after birth, for the newborn.10–12 It should also be noted that an increased risk of thrombosis, apparently reversible with control of hyperthyroidism, has recently been reported in endogenous hyperthyroidism.13 Because of the increased risk of deep venous thrombosis and pulmonary thromboembolism during pregnancy,14 a high level of suspicion should be maintained to allow for early detection of this complication, and prophylactic measures may have to be considered.
Maternal–fetal impact of hyperthyroidism.
| Maternal |
| Miscarriage |
| Preeclampsia (multiplies risk×5)7 |
| Preterm delivery (multiplies risk×10)12 |
| Abruptio placentae |
| Heart failure (multiplies risk×20)12 |
| Thyroid storm (multiplies risk×10)12 |
| Venous thrombosis13 |
| Fetal |
| Low birth weight |
| Intrauterine growth retardation |
| Prematurity |
| Small for gestational age |
| Fetal death |
| Thyroid dysfunction (hyperthyroidism or hypothyroidism) |
| Fetal goiter |
| Neonatal |
| Transient hyperthyroidism |
| Transient or permanent hypothyroidism |
Table 3 describes the different diagnostic possibilities when hyperthyroidism occurs during pregnancy. On the one hand, there are a number of conditions directly resulting from pregnancy itself, which may be encompassed under the heading of transient gestational thyrotoxicosis (TGT). On the other hand, any condition leading to thyroid hyperfunction outside pregnancy may also occur during pregnancy. Finally, increased TH levels may also occur due to exogenous TH supply.
Etiological classification of hyperthyroidism in pregnancy.
| Transient gestational thyrotoxicosis |
| Hyperemesis gravidarum |
| Multiple pregnancy |
| Trophoblastic hyperthyroidism (mole, choriocarcinoma) |
| Hyperreactio luteinalis |
| Familial gestational thyrotoxicosis |
| Hyperplacentosis |
| Thyroid disease |
| Graves disease |
| Thyroiditis |
| Multinodular goiter |
| Toxic thyroid adenoma |
| Iatrogenic |
| Overtreatment |
| Inadvertent intake of thyroid hormones (food contamination, etc.) |
| Factitious |
This is the most common cause of hyperthyroidism during pregnancy, and may occur in 1–3% of all pregnancies.15,16 It has been defined as a transient hyperthyroidism which is limited to the first half of pregnancy and characterized by increased FT4 or TT4 (adjusted) levels with suppressed or undetectable TSH in the absence of thyroid autoantibodies16 or physical features suggesting Graves’ Disease (GD).17 This is usually the result of increased HCG levels or from a greater affinity for TSH receptors.
The most characteristic condition within this group (Table 3) is hyperemesis gravidarum (HG), but there are other conditions also associated with increased HCG levels such as multiple pregnancy or trophoblastic disease (hydatid mole or choriocarcinoma) with a TGT prevalence up to 50%.17 There are also other less common causes such as hyperreactio luteinalis,18 characterized by the formation of theca-lutein cysts in the setting of pregnancy, or hyperplacentosis,19 where increased placental weight and HCG production are seen. There is also a familial condition (familial gestational thyrotoxicosis20) where hypersensitivity of the TSH receptor to physiological HCG levels occurs due to a dominant autosomal mutation; this is clinically characterized by TGT development in all pregnancies and all women in the family with normal HCG levels.
Hyperemesis gravidarumThe most common cause of TGT is HG. HG occurs in 0.5–10/1000 of all pregnancies,21 and is associated with increased free TH levels and TSH suppression in 30–60% of cases.22 It should be noted that the concept of HG should be restricted to conditions associated in the first trimester of pregnancy with vomiting, dehydration, loss of at least 5% of body weight, and ketonuria.23
In these cases, hyperthyroidism is characterized by the suppression of TSH levels and minimum FT4 increases, with commonly normal FT3 levels. Thyroid hyperfunction, as well as associated vomiting, usually resolve spontaneously before week 20, and symptomatic treatment with intravenous hydration and vitamin B complex is sufficient to prevent the exceptional risk of Wernicke's encephalopathy.24 There is no evidence that treatment with anti-thyroid drugs (ATD) provides any benefit,25 and their use is therefore inadvisable.6
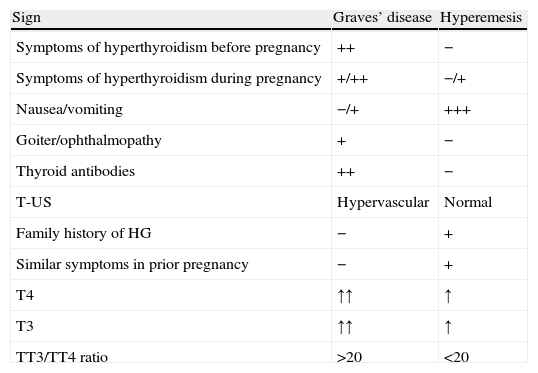
Exceptionally, HG with a highly conspicuous thyroid hyperfunction component or GD with associated gastrointestinal intolerance (nausea and vomiting) may suggest the need for differential diagnosis between them. Table 4 shows the main differences. In any case, if hyperthyroidism lasts beyond the first trimester of pregnancy, a cause other than TGT should be suspected.
Differences between Graves’ disease and hyperemesis.
| Sign | Graves’ disease | Hyperemesis |
| Symptoms of hyperthyroidism before pregnancy | ++ | − |
| Symptoms of hyperthyroidism during pregnancy | +/++ | −/+ |
| Nausea/vomiting | −/+ | +++ |
| Goiter/ophthalmopathy | + | − |
| Thyroid antibodies | ++ | − |
| T-US | Hypervascular | Normal |
| Family history of HG | − | + |
| Similar symptoms in prior pregnancy | − | + |
| T4 | ↑↑ | ↑ |
| T3 | ↑↑ | ↑ |
| TT3/TT4 ratio | >20 | <20 |
T-US: thyroid ultrasound; HG: hyperemesis gravidarum.
Among thyroid diseases, GD is the most common cause of hyperthyroidism in pregnancy, occurring in 0.1–1% of all pregnancies (clinical and subclinical hyperthyroidism in 0.4% and 0.6% respectively).10
The natural history of GD during pregnancy is an exacerbation of symptoms during the first trimester due to the additive effect of stimulation by HCG of the TSH receptor, followed by an improvement during the second half of pregnancy due to the immunomodulatory effect of pregnancy, and its recurrence after birth.
Graves’ disease in pregnancyThe symptoms of active GD in pregnancy do not differ from those typical of the condition. Some symptoms may sometimes be confounded with those of pregnancy itself. The possible presence of ocular signs and goiter helps clarify the condition, and hormone measurements allow for diagnosis.
Treatment of Graves’ disease in pregnancyAs is well known, there are three potential therapeutic approaches to GD in non-pregnant women: 131I, surgery, or medical treatment with ATDs. Of these three options, the administration of 131I is contraindicated in pregnancy because of the risk of malformation,7 and options are therefore limited to thyroidectomy or the use of ATDs. As regards surgery, it should only be performed in exceptional circumstances, as discussed below.
Medical treatment. Antithyroid drugsFor medical treatment of GD in pregnancy, Propylthiouracil (PTU) was traditionally considered as the treatment of choice as compared to Methimazole/Carbimazole (MM/CM) until a few years ago.26 This concept was based on some studies suggesting the minimal placental transfer of PTU as compared to MM/CM27 and on the teratogenic effects of MM/CM. It is currently known that both ATDs cross the placenta and have therefore the same chance of affecting the fetus and causing fetal hypothyroidism.26
In addition, the role of MM/CM in the occurrence in the fetus of aplasia cutis and choanal and esophageal atresia, together with some other malformations globally called “methimazole embryopathy”,28 has been highly controversial,29–31 and these malformations have been attributed in some cases to the harmful effects of hyperthyroidism itself. Recent studies,32,33 however, show that MM/CM has teratogenic effects, unlike PTU.
On the other hand, reports of cases of fulminant toxic hepatitis in patients treated with PTU have appeared in recent years,34,35 with prevalence rates ranging from 0.1% to 0.5% and a high mortality rate (25–50%).10,36 Cases of neonatal hepatitis in newborns born to mothers who had been treated with PTU have even been reported.37,38
We therefore face a dilemma: on the one hand, it appears to be clearly recognized that MM/CM involves a risk of malformation, and on the other hand, that PTU may cause fatal complications.
When faced with this alternative, some authors have recommended ablation therapy before pregnancy in women with GD.39 However, this solution barely resolves the problem, because women with GD do not plan pregnancy in most cases. On the other hand, this measure does not prevent eventual fetal hyperthyroidism (Fhyper) due to the persistence of high levels of thyroid-stimulating antibodies (TSI), which requires medical treatment, so raising the problem again.
For this reason, the ATA set out criteria for the use of PTU in the general population and in pregnant women.40 For pregnancy, the ATA advises against the use of MM/CM in the first trimester of pregnancy (the organogenesis period) and recommends the use of PTU. After week 12 of pregnancy, PTU should be discontinued because of the risk of liver disease, and GD control should be continued with MM/CM. Outside pregnancy, the use of PTU is only recommended in two circumstances: first, if thyroid storm occurs, and second, in the presence of adverse effects induced by MM/CM.
To avoid a delay in the start of treatment with PTU, it is advisable for women with GD who may potentially become pregnant and who do not use any contraceptive method to have PTU available at home and to be instructed to switch medication (from MM/CM to PTU) as soon as pregnancy is verified.
Medical treatment. Clinical managementAs discussed above, treatment of GD during pregnancy should consist of PTU in the first trimester and MM/CM thereafter. The starting dose may range from 50 to 300mg/day of PTU in three divided doses, 5 to 15mg/day of methimazole, or 10 to 15mg/day of carbimazole as a single dose.6 An attempt should always be made to use the lowest possible dose.
Beta-blockers should only be used transiently, because their long-term use has been associated with intrauterine growth retardation, bradycardia, and neonatal hypoglycemia.41 Moreover, some authors have reported increased miscarriage with combined propranolol and ATD treatment.42
When adjusting ATD dose, an attempt should be made to maintain the thyroid function of the mother near the limit of subclinical hyperfunction, because the fetal thyroid gland is much more sensitive to the blocking effect of ATDs. In fact, the presence of detectable TSH is an indication that the ATD dose should be decreased.10,43
GD during pregnancy shows a highly dynamic course, so that up to 20%-30% of patients achieve a degree of control allowing for ATD discontinuation in the last trimester of pregnancy.44 The use of a combined scheme (ATDs and THs) for the treatment of GD is absolutely contraindicated in pregnancy because it causes fetal hypothyroidism (Fhypo).6,43
SurgeryAs regards the use of surgery to control GD in pregnant women, the different consensuses and clinical guidelines6,7,43 agree in recommending it only if the following occur:
- 1
Adverse reactions to ATDs which prevent their use.
- 2
The need for high ATD doses.
- 3
Patient noncompliance with medical treatment.
In addition, Italian guidelines43 also recommend surgery for cases of extensive maternal goiter with airway compression.
If performed, the best time for surgery is from the second trimester onwards. This warrants the use of beta-blockers and sodium iodide (50–100mg/day)45 for a short time period (10–14 days) in pregnant women with GD as a preparation for surgery. As discussed above, long-term treatment with beta-blockers should be avoided. Caution should also be taken when potassium iodide is administered to prevent the development of goiter and/or Fhypo.
Surgery causes a profound change in the situation. On the one hand, hyperthyroidism is resolved in pregnant women, but hypothyroidism is induced in the mother, requiring rapid replacement and regular TSH monitoring. TSI levels should also be controlled, and fetal hyperthyroidism should be monitored if required.
Changes in thyroid autoimmunity during pregnancySome authors have shown, at least in some cases, that the functional activity of antibodies against the TSH receptor changes from a stimulating to an inhibitory or blocking activity.46,47 This means that pregnancy not only modulates autoantibody levels, but also their functional profile. This concept implies that GD in pregnancy may evolve not only to normal function, but even to hypofunction because of this change in stimulating-blocking activity, as has been reported in some cases.48 That is, GD in pregnancy is a changeable process that requires constant regular monitoring, and which should not be neglected even when a situation of apparent remission not requiring ATDs has been reached.
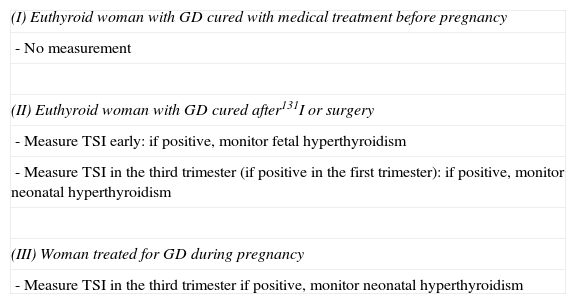
Measurement of thyroid-stimulating antibodies in pregnancyIn order to prevent the impact of potential TSI autoantibodies on the fetal thyroid gland, in 1998 the ETA published49 criteria establishing the indications for TSI measurement in pregnancy depending on the characteristics of GD in the mother (Table 5).
Indications for the measurement of TSI according to the European Thyroid Association.
| (I) Euthyroid woman with GD cured with medical treatment before pregnancy |
| - No measurement |
| (II) Euthyroid woman with GD cured after131I or surgery |
| - Measure TSI early: if positive, monitor fetal hyperthyroidism |
| - Measure TSI in the third trimester (if positive in the first trimester): if positive, monitor neonatal hyperthyroidism |
| (III) Woman treated for GD during pregnancy |
| - Measure TSI in the third trimester if positive, monitor neonatal hyperthyroidism |
GD: Graves’ disease.
Source: Laurberg et al.49
In cases where GD has been cured before pregnancy with medical treatment, the immune condition is considered to be resolved, and TSI measurement is not required.
When GD is resolved using ablation therapy (surgery or radiotherapy), the immune condition may persist. TSI levels usually become negative 12–18 months after surgery. After 131I ablation therapy, levels initially increase in the first few months and subsequently decrease, although they may remain high for up to five years.50 This means that in order to prevent potential Fhyper after 131I, long-term contraception has to be maintained. Thus, in women who want to conceive and have high TSI titers, surgery is preferred to 131I. In all these cases, TSI levels should be quantitated early to assess the potential risk of HF, and should be measured again in the third trimester to see if they continue to be positive and if a risk of neonatal hyperthyroidism therefore exists.
In the event of active GD during pregnancy, the potential risk of Fhyper is controlled by treatment of the mother with ATDs. It is however considered advisable to test TSI at the end of pregnancy to assess the risk of neonatal hyperthyroidism.
The ATA6 advises testing at approximately 20–24 weeks, and sets out much more general recommendations:
- -
Mother with active hyperthyroidism.
- -
History of treatment with 131I.
- -
Prior thyroidectomy.
- -
Prior newborn with hyperthyroidism.
It should be borne in mind that the fetal thyroid gland reaches maturity from week 20, and is therefore able to respond to the same influences (ATDs and TSI) as the adult thyroid gland. Because of this, both Fhypo and Fhyper may be found in the setting of GD in pregnancy.
Fetal hypothyroidismFhypo usually occurs due to relative ATD overdosage, which may maintain normal function in the mother, but causes a clear hypofunction in the fetus.
Suggestive clinical signsThe condition may clinically be suspected based on fetal goiter in obstetric ultrasound and in the development of polyhydramnios in a pregnant woman treated with ATDs.
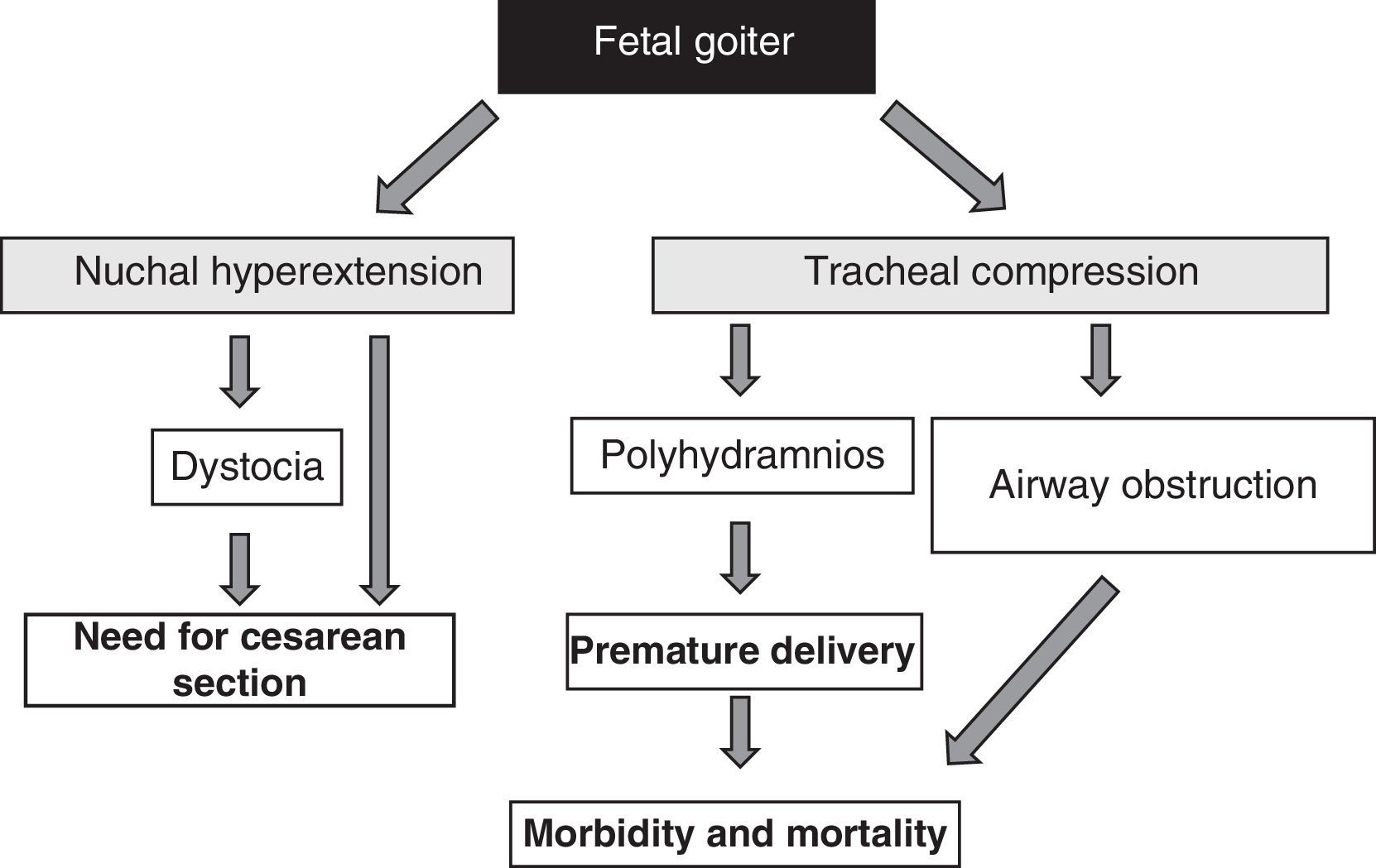
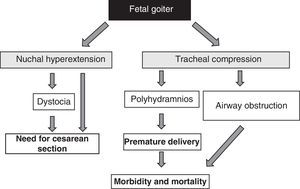
The presence of fetal goiter, irrespective of functional aspects, is an alarm signal because of its mass effect51 (Fig. 1). It should be taken into account that thyroid enlargement causes hyperextension of the back of the neck of the fetus, which in turn impairs intrauterine fetal mobility and, as a consequence, leads to an abnormal presentation at birth (shoulder dystocia, breech presentation, etc.). Even if this hyperextension does not prevent an adequate cephalic position of the fetus, it prevents adequate flexion of the fetal neck for delivery, causing face presentation. All these situations require the performance of a cesarean section. However, this is not the main complication, as compression exerted by goiter on the immature tracheal cartilages of the fetus can cause tracheal and esophageal compression. This results in airway obstruction, with its attendant risk of asphyxia. Moreover, esophageal compression impairs the swallowing of amniotic fluid, leading to fluid accumulation and the development of polyhydramnios. This favors premature birth, the main cause of neonatal morbidity and mortality.
DiagnosisThe only fully reliable procedure for confirming a suspected Fhypo is the measurement of fetal TH levels using chordocentesis. This is a procedure that is not devoid of complications (it is associated with a 2% risk of fetal death52) and is more complex than simple amniocentesis. However, the measurement of fetal TH levels in amniotic fluid is not reliable and does not correlate to their measurement in fetal blood.53 It should be borne in mind that THs of maternal origin may be found in amniotic fluid.54 According to the different guidelines,6,43 chordocentesis should only be performed when goiter is detected in a fetus whose mother is taking ATDs and a certain diagnosis of Fhyper or Fhypo cannot be made, and always at an experienced center.
Instead of this approach, the measurement of compound W, resulting from the metabolism of fetal THs, has recently been suggested. In the fetus, unlike in adults, the metabolism of THs follows the sulfo-conjugation pathway to form diiodothyronine sulphate; this compound is in turn methylated in the placenta and uterine wall before passing into maternal circulation and forming compound W. This compound is found in all pregnant women from the development of fetal thyroid function and gradually increases during pregnancy, disappearing after delivery. It has therefore been proposed as a marker of fetal thyroid function.55 In this setting, an inadequate progression of compound W levels in a pregnant woman treated with ATDs may suggest the development of Fhypo.56 However, for the time being this is no more than a quasi-experimental method.
TreatmentThe first measure to be taken is the discontinuation of ATDs. However, because of the latency of ATD effects once discontinued, TH replacement is also required until the fetal thyroid gland recovers completely. The placental passage of THs is limited, and they should therefore be administered by the intra-amniotic route. This route, though invasive, is much safer than chordocentesis, with a minimal risk of miscarriage after the first half of pregnancy.57
As regards intra-amniotic treatment of the fetus with T4, there is no established treatment scheme, and the literature data are quite conflicting.53,58,59 A recent review51 of cases reported to date concluded that the mean dose is approximately 279μg of levothyroxine once weekly (for one to six weeks). This appears to reverse fetal goiter 0.5–2.5 weeks after the first dose. Repeat TH measurements were made in some cases, while in others goiter disappearance was only monitored by ultrasound.
Fetal hyperthyroidismFhyper is a rare complication and may occur in up to 1–2% of babies born to mothers with current or prior GD, although it may possibly be an underdiagnosed condition.60,61
The development of Fhyper, except for the very rare cases of TSH receptor mutations,62 may be the result of poorly controlled GD in a pregnant woman or, more commonly, may arise in a pregnant woman with GD cured before pregnancy with ablation therapy who continues to have high TSI levels. The indications for measuring TSI to ascertain the risk of Fhyper have already been discussed. TSI levels 3- or 5-fold higher than normal6,63 involve a risk of Fhyper.
Suggestive clinical signsThe occurrence of fetal goiter is one of the earliest features. Fetal bradycardia, advanced bone age, overall growth retardation, and craniosynostosis may also be seen.64 In more severe forms, heart failure with fetal hydrops or impaired maturation of the central nervous system with mental retardation may occur. Premature delivery may eventually occur.65
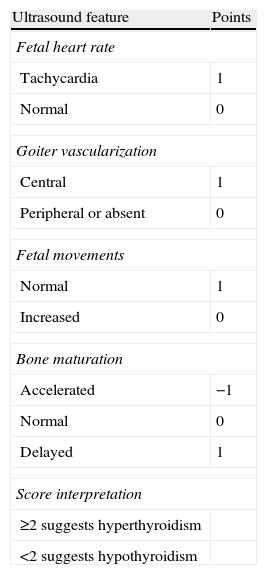
DiagnosisThe abovementioned signs are not definitive; tachycardia does not always occur, and the presence of goiter in ultrasound is a common finding with Fhypo. If doubt arises as to whether the condition is Fhypo due to the effect of ATDs or Fhyper due to poor maternal control, some authors have proposed a scoring system based on ultrasound data66 (Table 6). If doubt persists, fetal THs should be measured using chordocentesis, as stated in the Fhypo section. Compound W (previously discussed) may possibly be helpful for this purpose in the future.
Ultrasound scoring to differentiate hyperthyroidism from hypothyroidism in fetal goiter.
| Ultrasound feature | Points |
| Fetal heart rate | |
| Tachycardia | 1 |
| Normal | 0 |
| Goiter vascularization | |
| Central | 1 |
| Peripheral or absent | 0 |
| Fetal movements | |
| Normal | 1 |
| Increased | 0 |
| Bone maturation | |
| Accelerated | −1 |
| Normal | 0 |
| Delayed | 1 |
| Score interpretation | |
| ≥2 suggests hyperthyroidism | |
| <2 suggests hypothyroidism | |
Treatment should be based on ATDs. In this case, unlike for Fhypo, treatment should not be administered by the intra-amniotic route because ATDs freely cross the placenta. In the event of Fhyper in a pregnant woman with poorly controlled GD, only treatment adjustment in the mother is required. By contrast, if the mother has a normal function thanks to prior ablation therapy, and only the fetus shows hyperfunction, MM/CM should be administered to the mother starting with doses of 10–20mg/day. Treatment should be monitored every 4–5 days based on fetal HR and the course of goiter in order not to overdose the fetus and cause Fhypo.10
If the mother experiences hypothyroidism when ATDs are administered, she should be given levothyroxine, which barely crosses the placenta and therefore compensates for hypothyroidism without affecting the fetus. This is the only circumstance in which the combined scheme can be used.
Graves disease in the newbornIn newborns, GD during pregnancy may cause both hyperthyroidism and hypothyroidism.
Neonatal hyperthyroidismThis may occur in 1–5% of babies born to pregnant women with active GD10 and high TSI titers in the final trimester. The fetus maintains normal function during pregnancy due to the effect of the ATDs received by the mother. ATDs have a half-life of 24–72h, and after this period cease acting on the fetal thyroid gland. This becomes exposed to maternal TSI, which may persist for up to 12 weeks after delivery.
Thyroid hyperfunction and even heart failure, occurring a couple of days after delivery in an initially asymptomatic newborn, appear as a result.
This condition may also occur in newborns to mothers who have received ablation therapy before pregnancy and continue to have elevated TSI levels, in whom undiagnosed Fhyper occurred. In these cases, neonatal hyperthyroidism is a continuation of Fhyper; newborns show severe involvement, low weight, and accelerated bone maturation at birth.
Treatment should consist of the administration of MM/CM at doses of 0.5–1mg/kg body weight/day and propranolol 2mg/kg body weight/day36 depending on the clinical course and bearing in mind that this is a transient situation. It should be noted that TSIs may eventually change their functional profile to become receptor blockers and develop hypothyroidism.
Secondary neonatal hypothyroidismIn babies born to mothers with poorly controlled GD, it may be found that elevated levels of THs to which they have been exposed during pregnancy have caused the suppression of TSH secretion leading to secondary hypothyroidism at birth.67 This situation may be transient or definitive as the result of permanent pituitary impairment in TSH secretion.
Newborns should be administered levothyroxine, followed by regular assessment until the eventual recovery of TSH secretion.
Transient neonatal hypothyroidism due to the persistence of maternal autoantibodies blocking the RSH receptor may also be seen.68
Graves disease in postpartum womenPreviously controlled GD, either before or after pregnancy, may recur after delivery. The recurrence rate after delivery may be up to 84%, as compared to 56% in women with no pregnancy.69 It is therefore recommended that maternal THs be measured six weeks after delivery and for up to one year.10
If GD recurs, medical treatment with MM/CM will be required. Although ATDs are secreted in breast milk, their administration involves no risk during lactation, and up to 20–30mg of MM/CM may be taken with no impact on the thyroid gland of newborns.70 ATDs should be taken in divided doses and always after the end of milk intake.
It has been suggested that TSI in breast milk could reach the general circulation of newborns through the immature gastrointestinal epithelium of the infant and cause or prolong neonatal hyperthyroidism. However, there is as yet no conclusive evidence that this occurs.71
AddendumAfter the submission of this manuscript, the North American Society of Endocrinology issued new clinical practice guidelines on the Management of thyroid dysfunction during pregnancy and postpartum (J Clin Endocrinol Metab 2012; 97: 2543–2565). The recommendations given in the section on hyperthyroidism in pregnancy do not substantially differ from those discussed in this review.
Conflicts of interestThe author states that he has no conflicts of interest.
Please cite this article as: Gargallo Fernández M. Hipertiroidismo y embarazo. Endocrinol Nutr. 2013;60:535–543.