The diagnosis of hypertension and the clinical decisions regarding its treatment are usually based on daytime clinic blood pressure (BP) measurements. However, the correlation between BP levels and target organ damage, cardiovascular (CV) risk, and long-term prognosis, is higher for ambulatory (ABPM) than clinic measurements, both in the general population as well as in patients with diabetes. Moreover, there is consistent evidence in numerous studies that the asleep BP better predicts CV events than either the awake or 24h means. The prevalence of abnormal BP pattern and sleep-time hypertension is extensive in diabetes, often leading to inaccurate diagnoses of hypertension and its therapeutic control in the absence of complete and careful assessment of the entire 24h, i.e., daytime and night-time, BP pattern. Accordingly, ABPM should be the preferred method to comprehensively assess and decide the optimal clinical management of patients with diabetes directed to properly reduce elevated sleep-time BP, which might also lead to a significant reduction of CV events.
Habitualmente, el diagnóstico de hipertensión y las decisiones clínicas para su tratamiento se basan en un número limitado de valores de presión arterial (PA) obtenidos en la consulta clínica. Sin embargo, la correlación entre el nivel de PA y el riesgo de daño en órganos diana y eventos cardiovasculares (CV) es mucho mayor para la monitorización ambulatoria de la PA (MAPA), tanto en población general como en pacientes con diabetes. Además, numerosos estudios independientes han demostrado que la media de PA durante el sueño es mejor marcador de riesgo CV que la PA clínica y que las medias de actividad o de 24h derivadas de la MAPA. La prevalencia de un patrón circadiano de la PA alterado y de hipertensión nocturna es muy elevada en pacientes con diabetes, por lo que en estos pacientes el diagnóstico de hipertensión y su control terapéutico son frecuentemente inadecuados en ausencia de valoración de la PA a lo largo de las 24h mediante MAPA. Por todo ello, la MAPA debe ser la herramienta de elección en pacientes con diabetes para el correcto diagnóstico de hipertensión y para establecer el esquema terapéutico más adecuado que permita el control de la PA nocturna elevada, lo que podría redundar a su vez en una reducción significativa de eventos CV.
Blood pressure (BP) shows a largely predictable circadian variation which results from the interrelationship of various physiological, neuroendocrine, and environmental factors: (i) behavioral changes associated with the activity-rest pattern; (ii) divergence from the light-darkness cycle in environmental temperature, humidity and noise, and (iii) endogenous circadian (∼24h) variation in neuroendocrine, endothelial, vasoactive, and hemodynamic parameters such as plasma norepinephrine and epinephrine (autonomic nervous system), atrial natriuretic peptide and calcitonin, renin, angiotensin and aldosterone (renin-angiotensin-aldosterone system; RAAS).1,2 Different circadian rhythms in physiological and biochemical functions also significantly affect the pharmacokinetics and pharmacodynamics of antihypertensive drugs, as has been widely reported.3–5
A diagnosis of hypertension and clinical decisions regarding its treatment are usually based on a limited number of BP values taken in the consulting room, occasionally complemented by home self-measurements, always during the activity cycle.6 However, the correlation between BP level and the risk of target organ damage and cardiovascular (CV) events is much greater for ambulatory BP monitoring (ABPM) in both the general population7,8 and in patients with diabetes.9,10 One of the additional advantages of ABPM is that it allows for the description and quantification of the circadian variation profile of BP.
In the last few decades, the value of various parameters estimated from ABPM as biomarkers or mediators of target organ damage and the risk of CV events has been explored. Specifically, various prospective studies of ABPM have shown an increased risk both of target organ damage and of the incidence of fatal and nonfatal CV events associated with decreased BP dipping (i.e. the percent decrease in BP during sleep compared to mean BP during the activity period) that characterizes subjects with non-dipper profile (systolic BP [SBP] dipping <10%) not only in hypertensive patients both without8,11–14 and with diabetes9,10,15–17, but also in normotensive subjects.18 In addition, many independent studies have shown that mean resting (sleep time) BP is a better marker of CV risk than the routine clinical BP and than the means during activity or 24h derived from ABPM,8,12–14,19–25 and that this also applies to patients with diabetes.10,17,26 These studies usually show that when activity and resting means adjusted for significant influencing variables (including sex, age, diabetes, chronic kidney disease, smoking, prior CV event, etc.) are jointly analyzed, only the resting mean, and not the activity mean, is a significant and independent marker of CV morbidity and mortality. This review presents new emerging insights into changes of BP circadian pattern in patients with diabetes and its potential normalization through timing (chronotherapy) of antihypertensive therapy at bedtime with the dual objective of increasing BP control and decreasing CV risk.8,10,27–30
Ambulatory blood pressure pattern in patients with and without diabetesPatients with diabetes are among the groups with the greatest interest in the potential of ABPM as a diagnostic tool because of the strong association between diabetes and an increased risk of target organ damage, stroke, and CV morbidity and mortality. The non-dipper pattern and nocturnal hypertension, conditions that require ABPM for diagnosis, are common in diabetes.31–37 The prevalence of the non-dipper pattern in diabetes reported in the medical literature is, however, highly inconsistent, ranging from 30% to 73%, possibly because of disparities between the different studies in the populations studied (treated vs untreated patients, patients with different clinical severity, etc.), relatively small sample sizes, the use of a single and therefore poorly reproducible 24h ABPM,38,39 and inadequate definition of the activity and resting periods using predefined time intervals for all the patients studied. In addition, several investigators have assessed the circadian BP pattern of patients with diabetes without the required comparison with non-diabetic subjects.
In one of the first studies on ambulatory BP in diabetes, Fogari et al.31 assessed the prevalence of the non-dipper profile in 96 patients with type 2 diabetes (48 normotensive and 48 hypertensive patients) and in 103 controls without diabetes (50 normotensive and 53 hypertensive patients). In contrast to the results of subsequent studies in much larger populations,40 mean BP values during 24h and during the activity period (arbitrarily defined as the time period from 06:00 to 22:00h, irrespective of the sleep/wake cycle of each patient) were equivalent in patients with and without diabetes. Mean resting BP (arbitrarily, from 22:00 to 06:00h) was slightly but not significantly higher in patients with diabetes as compared to those without diabetes, both normotensive and hypertensive. The prevalence of the non-dipper pattern was among the lowest reported to date, i.e. 30% and 31% in normotensive and hypertensive patients with diabetes respectively.
Cuspidi et al.34 used 24h ABPM to evaluate twice in a four-week period 36 treated hypertensive patients with long-standing diabetes (>10 years) and 61 untreated hypertensive patients with no diabetes. The prevalence of the non-dipper pattern was 63.9% and 36.3% in patients with and without diabetes respectively, although these results may have been biased due to the absence of antihypertensive treatment in the group of non-diabetic patients. The authors also documented that (i) intraindividual variability of the non-dipper pattern is lower in patients with diabetes; (ii) patient classification as dipper or non-dipper based on a single 24h ABPM recording is more reliable in patients with diabetes, and (iii) the most common and reproducible non-dipper profile in patients with diabetes is associated with a greater prevalence of target organ damage.
Pistrosch et al.36 reported an even greater prevalence of the non-dipper, 73%, in 107 hypertensive patients with diabetes evaluated a single time with 24h ABPM. These authors also concluded that the change in circadian BP variation is more related to postprandial hyperglycemia than to basal fasting hypoglycemia.
Afsar et al.35 found a 56.3% prevalence of the non-dipper profile, defined by them as a <10% dipping in both systolic BP (SBP) and diastolic BP (SBP), in 96 hypertensive patients with diabetes assessed using 24h ABPM. The requirement to include a <10% dipping in DBP most likely caused a significant decrease in the actual prevalence of the non-dipper profile, which is often more adequately defined as an inadequate decrease in SBP only. Since the range of variation during the 24h is markedly different for SBP and DBP and the definition of a no-dipper profile is based on an arbitrary threshold of the percent difference between the mean BP values during the activity and resting periods, such a threshold cannot be the same for SBP and DBP in any case.41 Afsar et al.35 also concluded that the circadian variation profile of BP is related to insulin resistance and, thus, the Homeostatic Model Assessment (HOMA) index of insulin resistance may be a predictor of the non-dipper profile in hypertensive patients with diabetes.
Ayala et al.40 have recently investigated the impact of diabetes on the circadian profile of BP in hypertensive patients recruited into the Hygia Project, a multicenter, prospective, controlled ongoing study conducted by 292 investigators through a network of 40 health care centers (mostly primary care centers) in Galicia. This study has been designed to assess the prognostic value of ABPM and the impact of the time of antihypertensive treatment on CV, cerebrovascular, metabolic, and renal risk.40,42–46 The sample is representative of a population of hypertensive patients aged ≥18 years with a regular routine of daytime activity and nighttime rest. Using the inclusion and exclusion criteria specific for this cross-sectional study, the authors identified 12,765 hypertensive patients (6797 male and 5968 female) aged 58.1±14.1 (mean±SD) years (range, 18–97 years) who completed the study and provided all the information required. Among the participants, 2954 (1799 males and 1155 females had type 2 diabetes mellitus defined as basal blood glucose ≥126mg/dL in at least two measurements taken ≥3 months or glucose lowering treatment.47 At the time of assessment, 525/3314 patients with/without diabetes were not taking antihypertensive treatment, while the remaining 2429/6497 patients with/without diabetes were receiving treatment. BP was assessed automatically every 20min between 7:00 and 23:00h and every 30min during the night over a 48h period to increase the reproducibility of the results.38,39 The participants completed a diary recording their bedtime at night, waking up time in the morning, meal times, practice of physical exercise and episodes of unusual physical activity, altered emotional states, and other events that could affect BP. Ambulatory BP was considered to be controlled if the mean SBP/PBP in activity and at rest was <135/85 and <120/70mmHg respectively.6,41
Hypertensive patients with diabetes were predominantly older males diagnosed with albuminuria, chronic kidney disease, sleep obstructive apnea or obesity, and had higher creatinine, uric acid, and triglyceride levels, but lower total cholesterol and estimated glomerular rate values than patients without diabetes.40 Clinical SBP was significantly greater and DBP was significantly lower in patients with diabetes. As a consequence, a marked difference was found between the groups in pulse pressure (PP, the difference between SBP and DBP) measured at the clinic, which was significantly greater in patients with diabetes even after the results had been adjusted for age (p<0.001). The proportion of patients with PP ≥65mmHg and, thus, with greater CV risk,48 was significantly greater in patients with diabetes than in patients with no diabetes, 57% vs 35% (p<0.001).40
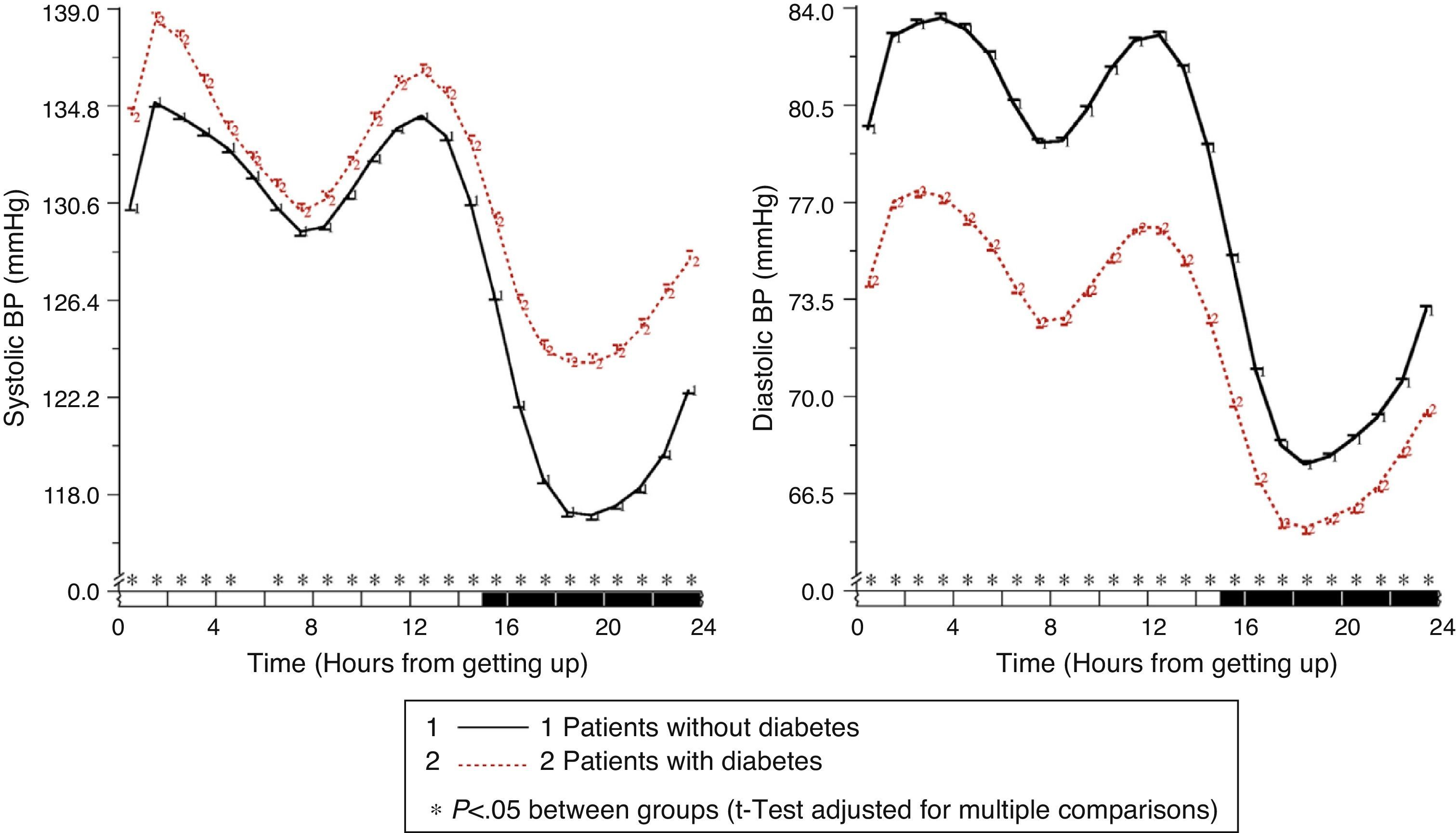
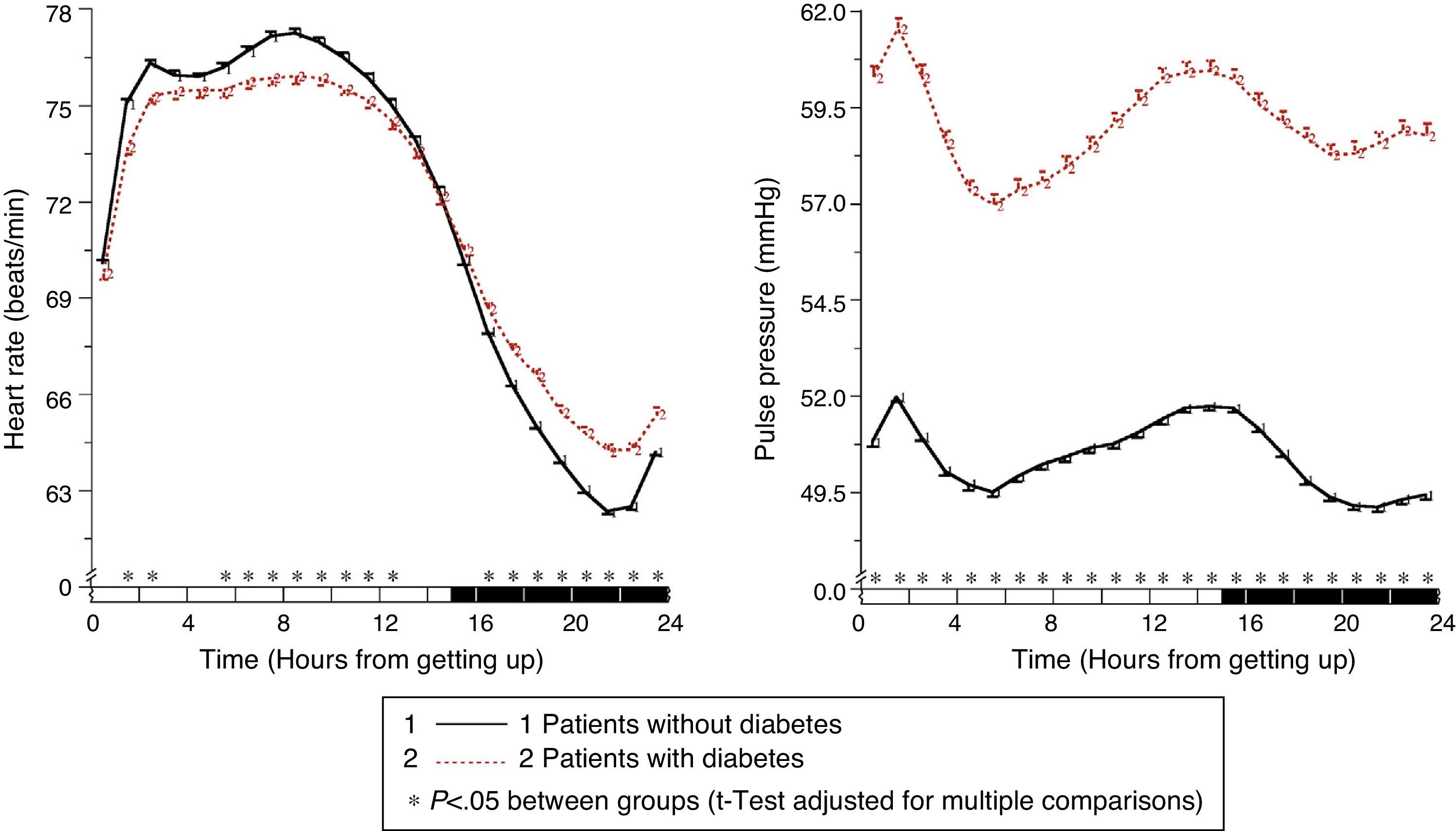
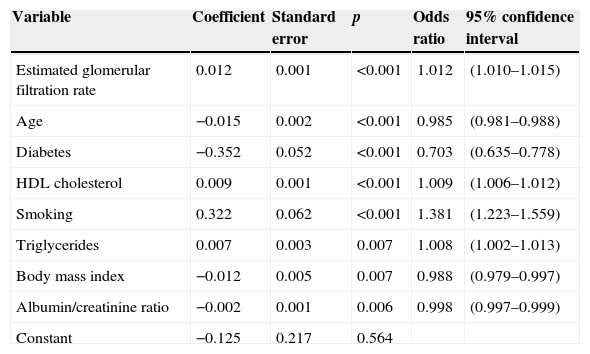
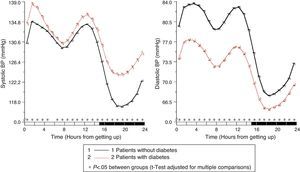
In patients with diabetes, ambulatory SBP was significantly higher (p<0.001), mainly during nighttime rest and in the first few hours of the activity cycle, regardless of the presence or absence of antihypertensive treatment (Fig. 1, left panel). Ambulatory BP was however significantly lower (p<0.001) in patients with diabetes, mainly during the daytime activity period (Fig. 1, right panel). As a consequence of these differences in SBP and DBP, ambulatory PP was significantly greater (p<0.001) in patients with diabetes throughout the 24h of the day (Fig. 2, right panel). The proportion of patients with a mean 48h PP >53mmHg, a threshold associated with greater CV risk,49 was significantly higher in patients with diabetes (63% vs 34%; p<0.001). Heart rate was significantly higher during nighttime rest and lower during most of the activity cycle in patients with diabetes as compared to those without diabetes (Fig. 2, left panel). The prevalence of the non-dipper pattern was significantly higher in patients with diabetes (62.1% vs 45.9%; p<0.001), as has recently been confirmed.50 The greatest difference between the groups was found in the prevalence of the riser pattern (<0% SBP dipping; 19.9% vs 8.1%; p<0.001). The main factor in the diagnosis of hypertension or inadequate BP control in patients with diabetes was elevated BP during sleep; thus, 89.2% of hypertensive patients with uncontrolled diabetes had nocturnal hypertension.40
Circadian SBP (left) and DBP (right) patterns in hypertensive patients without (continuous line) and with diabetes (dotted line) assessed using 48h ABPM. The shaded bar in the horizontal axis of the plots indicates the mean nighttime resting time of patients.
Circadian pattern of heart rate (left) and PP (right) in hypertensive patients without (continuous line) and with diabetes (dotted line) assessed using 48h ABPM. The shaded bar in the horizontal axis of the plots indicates the mean nighttime resting time of patients.
In addition, Ayala et al.40 used the data collected from the 12,765 participants in their cross-sectional study to investigate potential factors influencing the non-dipper profile in hypertensive patients. Logistic regression analysis indicated that the non-dipper profile (as categorical variable) was simultaneously and significantly associated, in decreasing importance, with a decreased estimated glomerular filtration rate, advanced age, the presence of diabetes, low HDL cholesterol, no smoking (due to the expected increase in BP during the activity period associated with the pressor effect of smoking), low triglycerides, an elevated body mass index, and an elevated albumin/creatinine ratio (Table 1). The non-dipper effect was significantly associated with an increase in antihypertensive drugs in a single morning dose. These results suggest a strong association between the absence of an adequate BP decrease during sleep (non-dipper pattern) and diabetes, the presence of renal disease, aging, and central obesity.
Logistic regression model of the circadian profile (dipper/non-dipper) of ambulatory BP in hypertensive patients.
| Variable | Coefficient | Standard error | p | Odds ratio | 95% confidence interval |
|---|---|---|---|---|---|
| Estimated glomerular filtration rate | 0.012 | 0.001 | <0.001 | 1.012 | (1.010–1.015) |
| Age | −0.015 | 0.002 | <0.001 | 0.985 | (0.981–0.988) |
| Diabetes | −0.352 | 0.052 | <0.001 | 0.703 | (0.635–0.778) |
| HDL cholesterol | 0.009 | 0.001 | <0.001 | 1.009 | (1.006–1.012) |
| Smoking | 0.322 | 0.062 | <0.001 | 1.381 | (1.223–1.559) |
| Triglycerides | 0.007 | 0.003 | 0.007 | 1.008 | (1.002–1.013) |
| Body mass index | −0.012 | 0.005 | 0.007 | 0.988 | (0.979–0.997) |
| Albumin/creatinine ratio | −0.002 | 0.001 | 0.006 | 0.998 | (0.997–0.999) |
| Constant | −0.125 | 0.217 | 0.564 |
Odds ratio with 95% confidence intervals for systolic BP dipping as a discrete variable (0=non-dipper, 1=dipper), calculated for each increase by 1mL/min/1.73m2 in estimated glomerular filtration rate; each year of age increase; each mg/dL increase in HDL cholesterol; each 10mg/dL increase in triglycerides; each kg/m2 increase in the body mass index; and each increase by 10mg/gCR in the albumin/creatinine ratio. Diabetes, defined as 0=no, 1=yes. Smoking, defined as 0=no, 1=yes. The glomerular filtration rate was estimated using the CKD-EPI equation.51 Model variables are listed in order of importance, established by the selection of variables using forward stepwise logistic regression analysis.
Hypertensive patients, including those with diabetes, usually take all their antihypertensive medication in the morning. It has been documented, however, that various circadian rhythms in physiological and biochemical functions and processes may significantly affect the pharmacokinetics (release, absorption, distribution, metabolism, and elimination processes) and pharmacodynamics (pharmacological effects) of antihypertensive drugs. Circadian time or time of administration of the drug during the 24h may therefore modify the pharmacokinetics or therapeutic and adverse effects of drugs.3–5
A large number of randomized clinical trials with antihypertensive drugs from six different classes (angiotensin-converting enzyme inhibitors [ACEIs], angiotensin II receptor blockers [ARBs], calcium channel blockers, α-blockers, ß-blockers, and diuretics) have documented relevant differences in their efficacy in decreasing BP, the duration of action, the safety profile, and their effects on the circadian BP pattern that depend on the time of day of their administration (chronotherapy).3–5 For example, the administration of ACEI or ARB monotherapy at bedtime, instead of after getting up in the morning, causes a greater decrease in BP during sleep with no loss of efficacy during the hours of activity, resulting in a significant increase in dipping toward a higher dipper profile. These results are also independent of the terminal half-life of the drug (which is usually only estimated from studies where patients were treated in the morning) and appear to be rather more related to RAAS activation during the second half of the sleep period.2 Recently, a greater BP decrease during sleep after intake at bedtime, as compared to morning administration, of various fixed combinations including captopril-hydrochlorothiazide (HCTZ), valsartan-amlodipine, fosinopril-amlodipine, olmesartan-amlodipine, amlodipine-HCTZ, and valsartan-HCTZ has also been consistently documented.4
Despite the availability of all this information, little research has been done on the impact of the administration time of antihypertensives on BP regulation in patients with diabetes. Tofé and García52 used a crossover design to assess in 38 hypertensive patients with type 2 diabetes mellitus the effects on ambulatory BP of the ARB olmesartan (40mg once daily for 8 weeks) taken first thing in the morning or at bedtime. Bedtime treatment, as compared to morning intake, resulted in a significantly greater decrease in mean resting SBP (−16.2 vs −11.8mmHg; p=0.007) and an increased BP dipping toward a higher dipper profile (7.4% vs 2.2%; p<0.001).
Moyá et al.42 recently investigated the impact of the administration time (as related to the rest/wake cycle of each subject) of antihypertensive treatment on the circadian profile and degree of control of ambulatory BP, and also on the clinical and laboratory parameters of interest, in hypertensive patients with diabetes using the cohort of patients participating in the Hygia Project described in the previous section.40 Among the 2429 hypertensive patients with diabetes on antihypertensive treatment evaluated (1465 males and 964 females aged 65.9±10.6 years), 1176 (700 males and 476 females) took all their medication in the morning, 336 (177 males and 159 females) took all their medication at bedtime, and 917 (588 males and 329 females) took the complete dose of some drugs at bedtime and the rest upon getting up in the morning. All the medication was administered once daily at the recommended therapeutic doses. In this study, the protocol did not allow for the dose of any antihypertensive drug to be split up and taken several times daily. Thus, patients who took a drug at bedtime ingested the complete dose at that time, and no part of the drug on getting up in the morning.
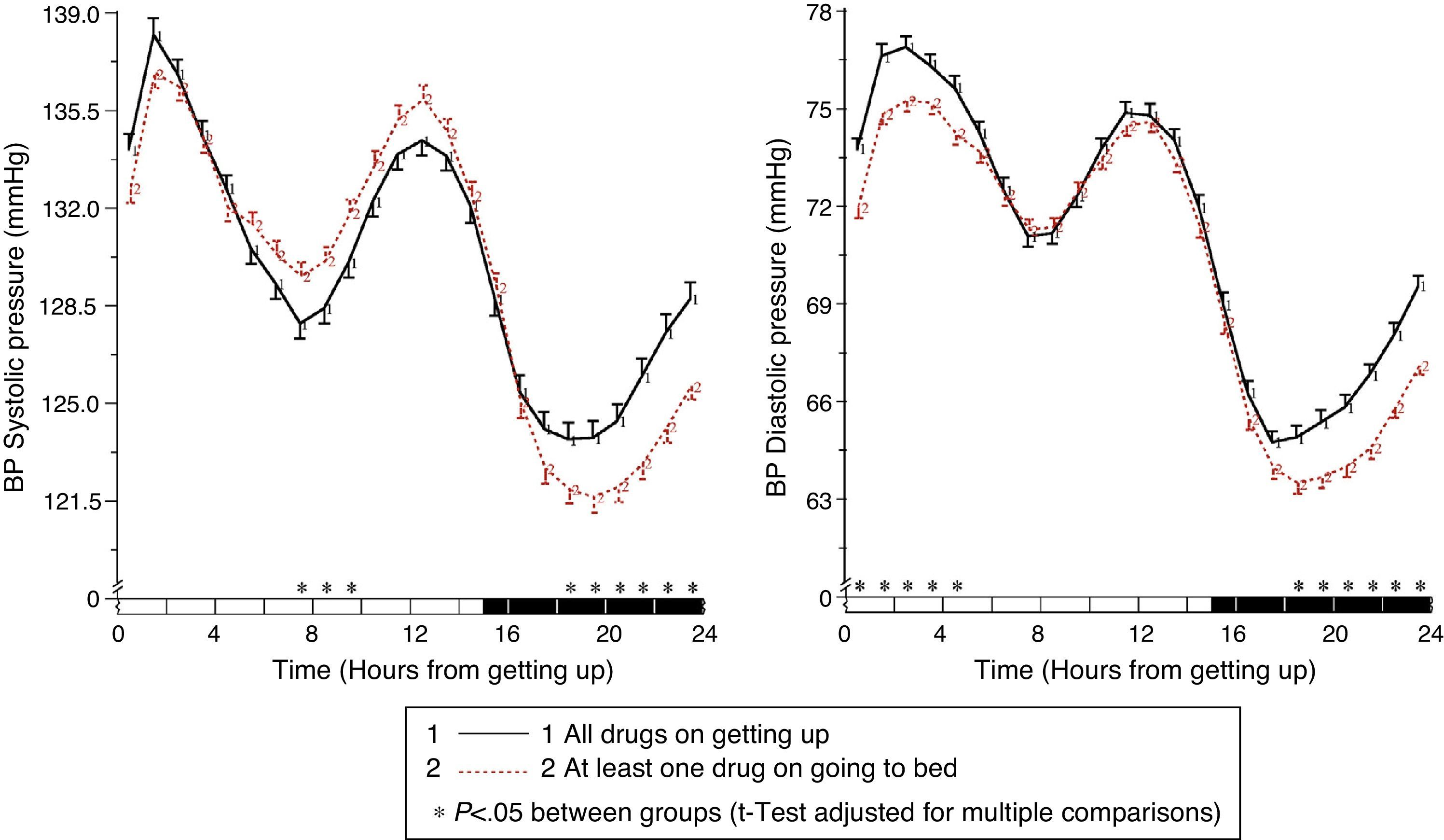
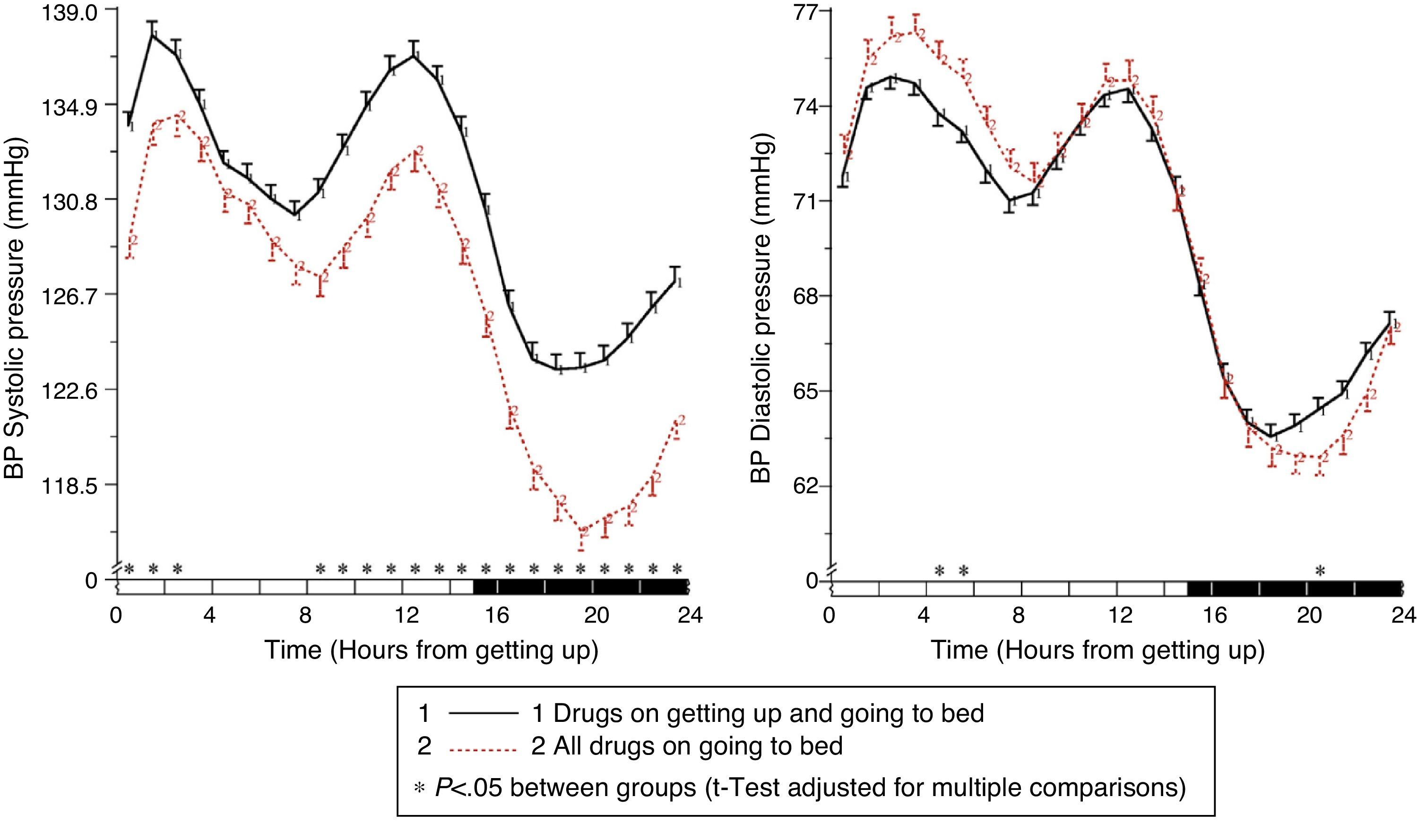
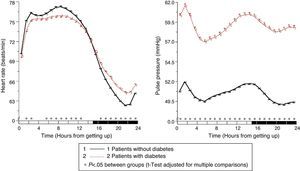
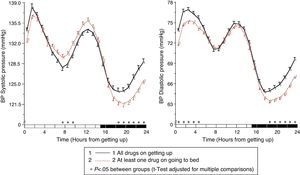
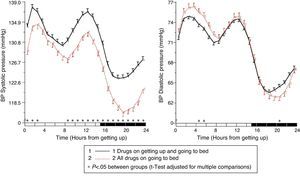
The results of the Moyá et al. study42 first suggested that patients with diabetes who took the complete dose of ≥1 antihypertensive drugs at bedtime had, as compared to those taking the whole medication upon getting up in the morning, a lower prevalence of metabolic syndrome and chronic kidney disease (49% vs 54%; p=0.023), a significantly lower albumin/creatinine ratio, glucose, total cholesterol and LDL cholesterol (p<0.001), and a significantly greater estimated glomerular filtration rate and HDL cholesterol (p<0.001). In addition, patients who took not only some, but all drugs at bedtime had, as compared to other treatment groups, the lowest glucose, creatinine and uric acid levels and a prevalence of proteinuria (5.5%) and chronic kidney disease (40%; p<0.001 as compared to all other groups). On the other hand, the intake of ≥1 antihypertensive drugs in full doses at bedtime was associated with a significantly lower mean resting BP than treatment with all medication upon getting up (p<0.001; Fig. 3). Dipping was significantly less and the prevalence of the non-dipper pattern was greater when all medication was taken upon getting up (68.6%) than when ≥1 drug was taken at bedtime (55.8%; p<0.001), and additionally decreased in patients who took the complete medication at bedtime (49.7%; p<0.001), because this latter group was characterized by having the lowest mean resting SBP (Fig. 4). The prevalence of the riser pattern was much greater (23.6%) in patients who took all their medication upon getting up in the morning as compared to those who took some (20.0%) or all drugs at bedtime (12.2%; p<0.001). The latter group had the highest rate of patients with well controlled ambulatory BP (p<0.001), which was achieved with a lower number of antihypertensive drugs (p<0.001) as compared to patients treated upon getting up.42
Circadian pattern of SBP (left) and DBP (right) in hypertensive patients with diabetes assessed using 48h ABPM and categorized based on their antihypertensive treatment scheme: intake of all medication in the morning (continuous line) or intake of the complete dose of ≥1 drug at bedtime (dotted line). The shaded bar in the horizontal axis of the plots indicates the mean nighttime resting time of patients.
Circadian pattern of SBP (left) and DBP (right) in hypertensive patients with diabetes assessed using 48h ABPM and categorized based on their antihypertensive treatment scheme: drug intake both in the morning and at bedtime (continuous line) or intake of the complete medication at bedtime (dotted line). The shaded bar in the horizontal axis of the plots indicates the mean nighttime resting time of patients.
Suzuki and Aizawa53 randomized 34 hypertensive patients with type 2 diabetes who were already receiving antihypertensive treatment into three groups in which 160mg of the ARB valsartan were added to the treatment regimen to be taken as follows: (i) the complete dose at breakfast; (ii) the complete dose at dinner, and (iii) half the dose (80mg) twice daily. The authors reported no differences between the three groups in the reduction of both clinical BP and BP measured at home during the activity cycle of the patients. The results of these and other studies on the potential effects of the administration time of antihypertensive drugs based on clinical or home BP assessment only are of little practical interest for various reasons. First, the “white coat effect” may compromise the validity of BP values measured in the consulting room, while inconsistent self-measurement procedures and low patient compliance may compromise BP measurements at home. Second, the selection of medication intake times was based on clock times rather than on the relevant biological information associated with the individual activity and resting cycle of each patient, as currently recommended.41 Third and most importantly, the research protocol of these studies did not include BP data collection over 24h in order to allow the clinically relevant characteristics of the circadian variation profile of BP most closely related to CV risk, particularly mean resting BP and BP dipping, to be established. Thus, the conclusions of those studies based on inadequate protocols not only have little scientific value, but may even be misleading, as they add confusion to the medical literature and cause corresponding and unnecessary controversy regarding the most adequate management of patients who require treatment.5
The impact of chronotherapy on cardiovascular risk in patients with and without diabetesMost ABPM studies conducted to date have many limitations including: (i) the use of arbitrary fixed time periods in clock hours to define wake/rest (or, erroneously, day/night), which leads to the calculation of values that do not represent the true mean activity and resting BP values of each subject; and (ii) the fact that most of the results reported come from studies based on a single ABPM recording in each patient at entry, under the apparently erroneous assumption that the ambulatory BP profile remains unchanged during the years of follow-up despite the effect of antihypertensive treatment, aging, and the development of target organ damage or concomitant diseases.8,14,24 Thus, the potential modification of CV risk associated with normalization of the circadian BP profile with antihypertensive treatment, i.e. the specific reduction in mean resting BP or increased dipping to a more reduced profile (dipper), remains controversial.
In this regard, the Ambulatory Blood Pressure Monitoring in the Prediction of Cardiovascular Events study (MAPEC) was designed to (i) prospectively investigate the comparative prognostic value of various parameters derived from ABPM and (ii) to assess whether intake of the complete dose of at least one antihypertensive drug at bedtime achieves better BP control and CV risk reduction than conventional therapy based on the administration of all medication upon getting up in the morning.8,14,23,27–30 This prospective study enrolled 3344 subjects, of whom 2610 were hypertensive patients based on ABPM criteria.6,41 Upon study entry and annually thereafter (or more frequently if antihypertensive treatment had to be adjusted based on ABPM results) for a median of 5.6 years of follow-up, BP and physical activity (wrist actigraphy) were simultaneously monitored for 48h for accurate and individualized measurement of mean activity and resting BP values. The results of the MAPEC study, the first and only study reported to date where the participants were regularly assessed by ABPM, suggest that mean resting, but not activity, SBP is the most significant predictor of CV events in a survival model adjusted for significant variables of sex, age, diabetes, anemia, and chronic kidney disease (per each 1-SD of elevation, hazard ratio [HR] 1.63; 95% CI [1.44–1.85]; p<0.001 for mean resting BP; 0.94 [0.81–1.08]; p=0.348 for mean activity BP). Assessment of the potential combined contribution of several parameters derived from ABPM as predictors of CV risk showed that the best adjusted model only included the mean resting SBP (HR=1.23; 95% CI [1.16–1.32]; p<0.001) and SBP dipping (HR=0.98; 95% CI [0.97–0.99]; p=0.019). In addition, when mean resting SBP was adjusted both for mean activity SBP and clinical BP, only the first significantly predicted for an increased risk of CV events in both the general population8,14 and specifically in patients with diabetes.10 More importantly, the analysis of changes in ambulatory BP during the years of follow-up showed a 17% decrease in CV risk per 5mmHg decrease in mean resting SBP irrespective of changes in clinical BP or mean activity calculated from ABPM.8,14,24 These results suggest that mean resting BP could be a new therapeutic target for CV risk reduction, which obviously requires accurate patient assessment with ABPM.41
On the other hand, the results of a small number of prospective clinical trials have allowed the impact of time of antihypertensive treatment on CV risk to be assessed. Thus, in the Syst.-Eur,54 Syst.-China,55 Heart Outcomes Prevention Evaluation (HOPE),56 and the Controlled Onset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE)57 studies, the tested drug (nitrendipine, ramipril, verapamil-COER) was administered at night. The purpose of CONVINCE was to decrease BP in the early morning, rather than during nighttime sleep. In fact, the delayed release verapamil formulation administered at bedtime decreased activity BP to a much greater extent than resting BP,58 so that if the therapeutic objective was to decrease mean resting BP, bedtime would not have been its most adequate administration time in any case. Despite this, a comparison of the results of those studies with drug intake at bedtime with those of 170 clinical trials where the tested drug was administered in the morning59 showed a 46% reduction (p=0.008) in the relative risk of CV events when antihypertensive medication was taken at bedtime as compared to early in the morning.60 Unfortunately, the drugs tested in those four trials with bedtime administration were not randomized, so it was not possible for the effects of the same medication taken in the morning to be assessed.
The MAPEC study is the first and so far the only prospective trial to date of the impact of antihypertensive chronotherapy on CV risk. In this study, patients randomized to drug intake at bedtime were characterized by having in their last ABPM, after 5.6 years of follow-up, lower mean resting BP, greater dipping, lower prevalence of the non-dipper pattern, and greater prevalence of controlled ambulatory BP as compared to patients who took all their medication upon getting up in the morning.27 Patients treated at bedtime had an HR of total CV events significantly lower than those treated in the morning (0.39; 95% CI [0.29–0.51]; p<0.001). The difference between the groups was also significant for all major events, i.e. the sum of CV death, myocardial infarction, and ischemic and hemorrhagic stroke (0.33; 95% CI [0.19–0.55]; p<0.001). These results were validated in subgroups with high CV risk and characterized also by a high prevalence of nocturnal hypertension, including patients with diabetes,28 refractory hypertension,23 and chronic kidney disease.29
ConclusionsThe ABPM studies reported to date and reviewed here agree in documenting a high prevalence of an altered circadian BP pattern in patients with diabetes. More importantly, the prevalence of the riser pattern, the BP pattern associated with the greatest CV risk, is more than double in patients with diabetes as compared to those with no diabetes. Patients with diabetes also have a significant elevation of ambulatory PP during the 24h, which reflects greater arterial stiffness and may therefore be an additional cause of the greater CV risk documented in them. One of the determinant characteristics of the BP profile in diabetes is the high mean BP during nighttime rest hours, which causes, in turn, a high prevalence of nocturnal hypertension and, as a result, an erroneous diagnosis of hypertension when this is only based on the clinical measurement of BP or even in home self-measurements. Overall, these results account for a great part of the increased CV risk in patients with diabetes and warrant the need to use ABPM as an indispensable tool in the diagnosis of hypertension in such patients, both for the adequate assessment of their CV risk and to determine the most adequate treatment scheme for controlling elevated nocturnal BP and ambulatory PP, which could in return result in a significant reduction of CV events, as has already been demonstrated.28 However, the unjustifiably high cost of currently marketed ABPM devices has often been argued as a potential limitation of ABPM,41 although the actual cost of ABPM per patient is currently half the cost of blood glucose measurement. An additional potential limitation of ABPM is patient tolerance throughout the day and night, especially given that ABPM may cause sleep disturbances in some patients.41 The degree of reproducibility of the circadian profile of BP in 24h ABPM recordings repeated in the same subjects with a relatively short time interval of only several weeks has also been reported as a potential limitation of ABPM. However, ABPM is markedly superior to clinical BP measurement in terms of reproducibility, especially if the monitoring period is extended to 48h, as has been clearly demonstrated.38,39
The goal of antihypertensive treatment is to decrease BP to prevent target organ damage and to decrease the risk of CV events. The benefits associated with BP reduction are consistent and, to a certain extent, independent of the medication used. Unfortunately, current therapeutic strategies are almost always focused on clinical BP reduction6 and do not allow for removing the risks associated with increased BP; by contrast, they allow for decreasing CV risk by approximately 33%, a clearly suboptimal result.61 A review of the incidence of CV events in the prospective studies reported shows that a relatively low level of major CV events could only be achieved in trials including hypertensive patients with low baseline CV risk, i.e. those excluding, amongst others, high-risk patients with diabetes, chronic kidney disease, or prior CV events.62 Moreover, the combined results of prior studies on CV morbidity and mortality which included patients from the abovementioned high-risk groups suggest that antihypertensive treatment is not able to adequately decrease CV risk, leading to the belief that such patients have a “residual risk” that cannot be decreased with standard treatment.63 This conclusion, not only questionable but also refutable,30 is based on the results of studies whose only therapeutic objective was to decrease standard clinical BP with drugs taken in a single daily dose.
This approach, still common, does not take into account (i) that the correlation between BP level and CV risk is much greater for ABPM than for clinical BP measures7–9; (ii) that mean resting BP, but not mean activity, 24h or clinical BP, is an independent prognostic marker of CV risk8,10,14,20–24; and (iii) that efficacy in decreasing the BP level (mainly mean resting BP) and in improving the circadian BP pattern toward a higher dipper pattern of a good number of antihypertensive drugs from six different classes and their combinations markedly depends on intake time in relation to the activity and resting cycle of the patient.3–5 In the specific case of diabetes, the results from the Moyá et al. study42 using data from the participants in the Hygia Project documented a significantly lower prevalence of CV risk markers and a better metabolic profile in patients with diabetes treated at bedtime as compared to those taking medication in the morning; the results also documented a lower mean resting BP and a decreased prevalence of the non-dipper/riser profile of high CV risk in patients with diabetes treated at bedtime. These results suggest that treatment at bedtime, together with ABPM assessment to make an accurate diagnosis of hypertension and to prevent potential nocturnal hypotension associated with treatment, should be the preferred treatment scheme in patients with diabetes.42
On the other hand, the results of the MAPEC study,8,14,23,27–30 pending their potential confirmation by other prospective studies such as the currently ongoing Hygia Project,40,42–46 suggest (i) that decreasing mean resting BP and increasing dipping toward a higher dipper profile –two new therapeutic objectives that require the evaluation of patients with ABPM–significantly decrease CV morbidity and mortality, and (ii) that the intake of the complete dose of at least one antihypertensive drug, and preferably all, at bedtime significantly decreases the risk of CV events in both the general hypertensive population27 and specifically in patients with diabetes.28 In this regard, it should be noted that the American Diabetes Association has recognized the clinical relevance of antihypertensive chronotherapy, recommending that hypertensive patients with diabetes be treated with ≥1 drug at bedtime.47 This recommendation implies that bedtime administration should be the treatment scheme of choice in all patients with diabetes with de novo diagnosis of hypertension. This same recommendation, complemented with the indication to use ABPM as the new gold standard for the diagnosis of hypertension and individualized assessment of CV risk, has recently been extended to other groups, including the elderly and patients with chronic kidney disease, a prior CV event, or refractory or secondary hypertension.41
Conflicts of interestThe authors state that they have no conflicts of interest. The authors assume full responsibility for the contents of this manuscript.
Please cite this article as: Hermida RC, Moyá A, Ayala DE. Monitorización ambulatoria de la presión arterial en diabetes para valoración y control de riesgo vascular. Endocrinol Nutr. 2015;62:400–410.