Cyanotic congenital heart disease (CCHD) refers to a group of heart diseases which occur after birth, affect 1/1000 live newborns, and are associated with systemic hypoxia. The incidence of congenital heart disease ranges from 12 to 14/1000 live newborns.1 The different congenital cardiac defects may cause increased pulmonary vascular resistance and pulmonary hypertension, so that approximately 8% of all congenital heart diseases and 11% of those with left-to-right shunts develop Eisenmenger's syndrome, characterized by progressive pulmonary vascular involvement and cyanosis resulting from systemic-pulmonary communication which causes shunt reversal. Eisenmenger's syndrome is the most common cause of CCHD in adults.2
Pheochromocytoma (PC) and paraganglioma (PG) are neuroendocrine tumors arising from chromaffin tissue which have a low incidence in the general population and a 0.2–0.6% prevalence in patients with high blood pressure. In more than 30% of cases, the occurrence of these tumors has been reported to be related to genetic changes.3 Some publications have reported the coexistence of both conditions, and a potential pathogenetic association has been postulated.
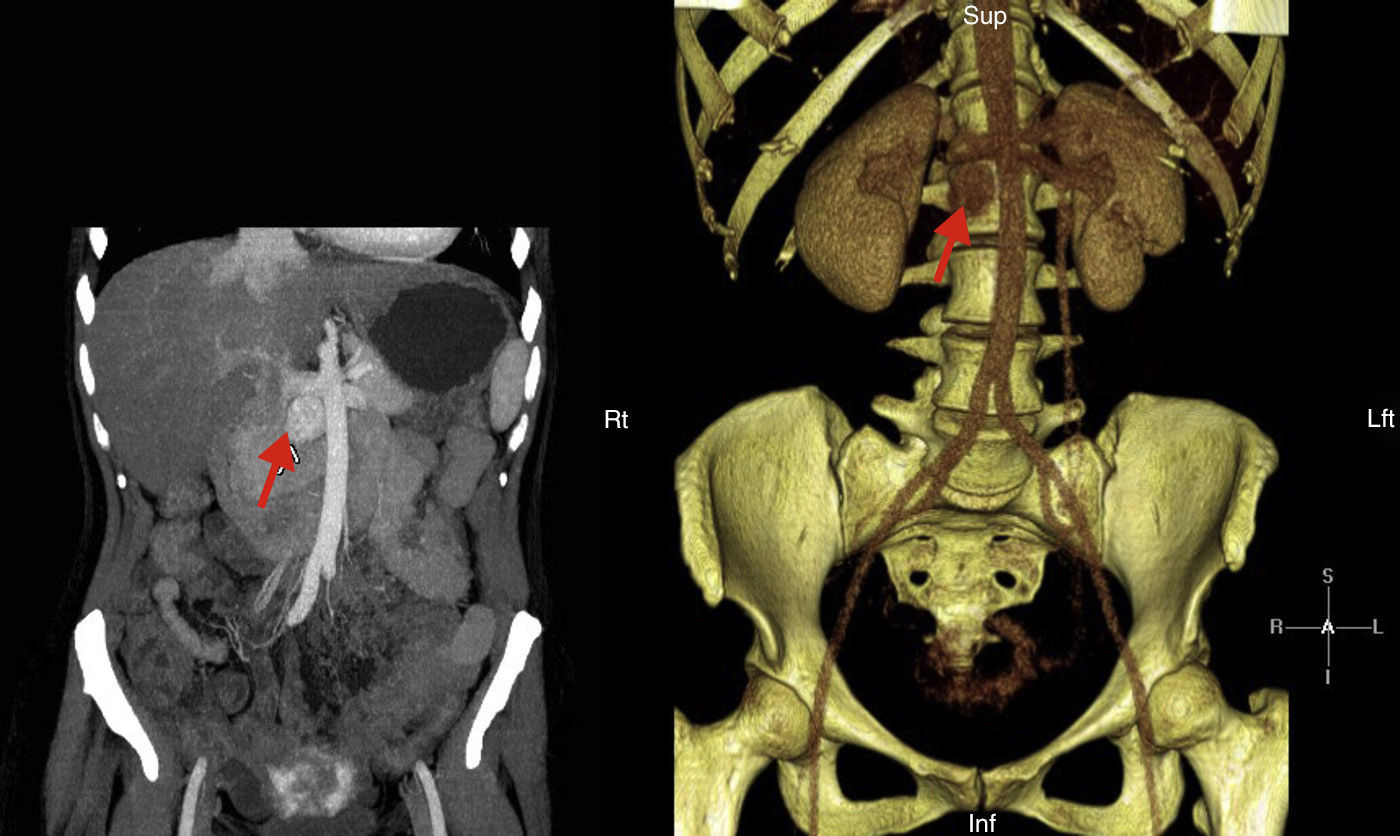
We report the case of a 41-year-old female patient diagnosed in childhood with congenital heart disease consisting of single double-chambered left ventricle with l-malposition of the great vessels and severe pulmonary hypertension in the Eisenmenger situation, with significant cyanosis and compensatory erythrocytosis, treated with sildenafil and bosentan. The diagnosis was made at five months of age and cardiac reconstruction surgery was rejected. The patient complained of HBP, asthenia, palpitations, and chest discomfort, and decompensated heart failure was found. Catecholamine hypersecretion was suspected, and hormone testing was performed with the following results: metanephrine level in 24h urine 215μg/24h (NV<341) and normetanephrine level 2491μg/24h (NV<444). An imaging study consisting of abdominal CT showed a retroperitoneal mass 3cm×2cm in size with a high contrast uptake in the interaortocaval space consistent with PG (Fig. 1). Adrenal gland scintigraphy with iodine-123MIBG and merging with CT images showed an area with pathological radiotracer uptake at the interaortocaval space coinciding with the lesion visualized by CT and consistent with the clinically suspected diagnosis. No distant lesions were shown. A molecular study was performed by sequencing encoding exons and exon–intron binding regions of genes: SDHD, SDHC, SDHB, VHL, SDHAF2, MAX, and TMEM127. No changes were found. After adequate alpha blockade with doxazosin, and under close cardiological monitoring, the patient underwent surgery. Histological examination of the surgical specimen revealed PG. No capsular or lymphovascular invasion was found. Immunohistochemistry revealed intense and diffuse expression for chromogranin, synaptophysin, and S100 staining in sustentacular areas; Ki-67 proliferation index: 1%. After surgery, urinary normetanephrine level was within the reference range, BP normalized, and there was an evident clinical improvement.
An association of hypoxia and genetic syndromes related to the presence of PC and PG (SDHx, von Hippel-Lindau, HIF2A) has been reported in recent years. Most of these syndromes lead to an aberrant activation of signaling pathways activating the synthesis of hypoxia-induced factors (HIF), responsible for the pathogenesis of PC and PG.4 It has been suggested that exposure to chronic hypoxia in patients with CCHD may increase the risk of developing PC and PG.5 The reported patient was diagnosed in childhood, so that the course of the disease involved a prolonged cyanosis. On the other hand, as regards the biochemical phenotype, only norepinephrine production was found. The value of the biochemical phenotype as a guide for performing the genetic study in patients with PC/PG has been reported in recent years. This has made it possible to differentiate two groups (clusters 1 and 2) with different signaling pathways altered. In cluster 1, associated with errors in abnormal HIF activation, an increased expression of angiogenic factors leading to tumor occurrence is seen. This cluster is characterized by having a noradrenergic phenotype with normal epinephrine secretion.6 Cluster 2 comprises a group of tumors caused by mutations in the rearranged during transfection (RET) proto-oncogene, the neurofibromatosis type 1 (NF 1) gene, and the TMEM127 gene with an adrenergic phenotype and predominant epinephrine secretion. The result of the genetic study performed in our patient ruled out a genetic predisposition. A diagnosis of PC/PG in these patients may be difficult to suspect due to the overlapping of symptoms. However, catecholamine hypersecretion may worsen the clinical picture, and it is therefore important to remember that the presence of PC/PG should be ruled out in patients with CCHD with a worsening of cardiac function.
Please cite this article as: Oleaga-Alday A, Goñi-Goicoechea F, Calles-Romero L, Pérez de Ciriza-Cordeu M, Paja-Fano M. Paraganglioma asociado a cardiopatía congénita cianótica: papel de la hipoxia tisular. Endocrinol Nutr. 2015;62:413–414.