Chlamydia trachomatis (C. trachomatis) is the most frequently reported sexually transmitted infection (STI) in developed countries, but there is a lack data on its incidence and population dynamics in Spain. The objectives of this study were to estimate the incidence of C. trachomatis in patients seeking medical attention in an STI clinic with a defined population catchment area, to identify factors associated with this infection, and to explore differences between factors associated with new infections and re-infections.
MethodsA retrospective study was conducted on a cohort of patients from a STI clinic who underwent chlamydia testing at least twice between 2007 and 2015.
ResultsOf the 2633 patients who met study selection criteria, 795 (30.2%) tested positive for C. trachomatis at baseline (baseline Chlamydia). The overall incidence was 7.97/100 person-years (95% CI: 7.2–8.8): 5.9/100 person-years (95% CI: 5.2–6.7) among patients testing negative for C. trachomatis at baseline, and 18.3 person-years (95% CI: 15.6–21.5) among those testing positive at baseline. In multivariate analysis, the factors independently associated with overall incidence were a history of infection with C. trachomatis in the previous 6 months (hazard ratio=3.6; 95% CI: 2.3–5.4), younger age (HR <20 vs ≥35 years=5.5; 95% CI: 3.2–9.5), male sex, 2 or more sexual partners in the previous month and year, and inconsistent condom use.
ConclusionsGuidelines should be established for C. trachomatis in Spain, including recommendations on the need for follow-up and re-testing, independently of age. Though data concerning the optimal timing of re-testing are inconclusive, our findings support the establishment of a 3–6 month interval.
Chlamydia trachomatis es la infección de transmisión sexual (ITS) más frecuentemente notificada en los países desarrollados, pero en España carecemos de información sobre su incidencia y su dinámica poblacional. Nuestros objetivos han sido estimar la incidencia de C.trachomatis en los pacientes de una clínica de ITS con una población de referencia definida, identificar factores asociados con ella y evaluar diferencias entre los factores asociados con las nuevas infecciones y las reinfecciones.
MétodosCohorte retrospectiva de pacientes de una Unidad de ITS con pruebas diagnósticas para Chlamydia en más de una ocasión entre 2007 y 2015.
ResultadosDe los 2.633 pacientes que cumplieron los criterios de inclusión en el estudio, 795 (30,2%) tuvieron un resultado positivo de C.trachomatis en el episodio basal (Chlamydia basal). La incidencia global fue de 7,97/100años-persona (IC95%: 7,2-8,8): 5,9/100 años-persona (IC95%: 5,2-6,7) entre los pacientes con chlamydia basal negativa y 18,3 años-persona (IC95%: 15,6-21,5) entre aquellos con chlamydia basal positiva. En los análisis multivariantes, los factores asociados independientemente con la incidencia global fueron haber padecido otra infección por C.trachomatis en los últimos 6meses (hazard ratio [HR]=3,6; IC95%: 2,3-5,4), menor edad (HR<20 vs ≥35=5,5; IC95%: 3,2-9,5), ser hombre, 2 o más parejas en el último mes o en el último año y la utilización inconsistente del preservativo.
ConclusiónSon necesarias guías de práctica clínica para C.trachomatis en España que incluyan recomendaciones sobre la necesidad de seguimiento y re-cribado, independientemente de la edad. El periodo óptimo para repetir las pruebas no está establecido, nuestros resultados apoyan la implantación de un intervalo de 3-6meses.
Chlamydia trachomatis (C. trachomatis) is the most frequently reported sexually transmitted infection (STI) in developed countries.1,2 Its incidence in the European Union was estimated to be 182 cases/100,000 population in 2013 (67% of the cases being among people aged 15–24 years old). It is likely that the real incidence is considerably higher, given underreporting due to asymptomatic infections and differences between countries in diagnostic practice and epidemiological surveillance systems. Among European Union countries that consistently reported cases of this condition between 2004 and 2013, the rate increased by an average of 68%,1 and the rate of reported cases in the USA increased 45% between 2000 and 2014.2 This growth is attributable to various factors: higher rates of unprotected sexual relations, increased detection and improved diagnostic tools.
Therefore, we are facing an increase in a condition that can cause severe complications and increases susceptibility to and transmissibility of HIV.3 Further, it mostly affects young people and is challenging to control, given the very high rate of asymptomatic infection, together with high rates of re-infection, ranging between 4.5 and 45 per 100 person-years,4,5 depending on the study population.
In Spain, it was not compulsory to notify cases of C. trachomatis to public health authorities until March 2015, and hence, we lack data on its incidence at the population level and the dynamics of the infection, and there are no organized chlamydia control activities. C. trachomatis prevalence studies in Spain are limited to specific populations: 4–8.5% in the general population under 25 years of age,6–8 4.3–6% among STI clinic patients,9,10 4.7–6% among sex workers,9,11 1% in parturient women in the Basque Country (6.4% in those under 25 years of age)12 and 7.25% in HIV men who have sex with men (MSM).13 We have not found C. trachomatis incidence studies in Spain except for López-Corbeto et al.’s study, which estimates a 10.3% re-infection incidence in a small sample of 29 women 16–25 years old.14
The objectives of this study were to estimate the incidence of C. trachomatis among patients of an STI clinic with a defined population catchment area, identify factors associated with C. trachomatis infection, and assess differences between the factors associated with new infections and re-infections.
Material and methodsThis was a retrospective cohort study carried out in patients of the STI Clinic run by the Infectious Diseases Department of Basurto University Hospital (Basque Health Service) who underwent more than one diagnostic test for C. trachomatis between January 2007 and July 2015. The catchment population of this clinic is the population of Bizkaia (over 1 million).
Since 1993, doctors have completed a standardized questionnaire for all patients seen in the clinic with information regarding their sociodemographic and clinical characteristics, as well as the results of diagnostic tests. These questionnaires are then scanned and the resulting electronic database was the source of data for this study.
The protocol for all patients included sample collection for C. trachomatis nucleic acid amplification tests in all body areas susceptible to infection depending on their sexual practices.
Until 2013, C. trachomatis DNA detection was performed using real-time polymerase chain reaction (PCR) with a Universal Probe Library TaqMan (Roche Diagnostics, Mannhein, Germany) probe that detects a specific region of the cryptic plasmid in the LightCycler 2.0 Instrument (Roche). During this period, DNA extraction was carried out using the NucliSENS easyMag (bio-Mérieux, Marcy l’Étoile, France). Since 2014, the diagnosis has been based on PCR analysis performed using the BD Max instrument (Becton Dickinson) with the probes and primers of the C. trachomatis and Neisseria gonorrhoeae Mix (Diagenode, Liège, Belgium) that also detect a specific region of the cryptic plasmid. For DNA extraction, the BD Max ExK DNA-1kit (Becton Dickinson, Quebec, Canada) has been used. Both assays target a plasmid sequence outside the deletion of Swedish variant of C. trachomatis.
Uncomplicated infections are treated with 1g of azithromycin. Proctitis and pelvic inflammatory disease are treated with doxycycline twice a day for 7–14 days. Medication is provided free of charge.
We do not perform a test-of-cure except in selected patients, that is, cases of persistence of symptoms, possible re-exposure, suspicion of poor treatment adherence or pregnancy, as recommended in clinical practice guidelines.15–17 In accordance with these guidelines, at the end of an episode of care, patients are requested to make an appointment for 3–6 months later for a re-testing, but we do not have an active recall system that sends reminders to return for STI re-testing.
For this study, we selected patients from whom samples for C. trachomatis testing had been taken on more than one occasion between January 2007 and July 2015, with a minimum interval between sample collections of 4 weeks.
The follow-up time for each patient was calculated from the date when the first sample was taken for C. trachomatis testing (baseline episode) to the following episode of C. trachomatis (a positive test result, in such cases), or until the date of the last negative test. All participants who tested positive for C. trachomatis at baseline had been treated with azithromycin or doxycycline.
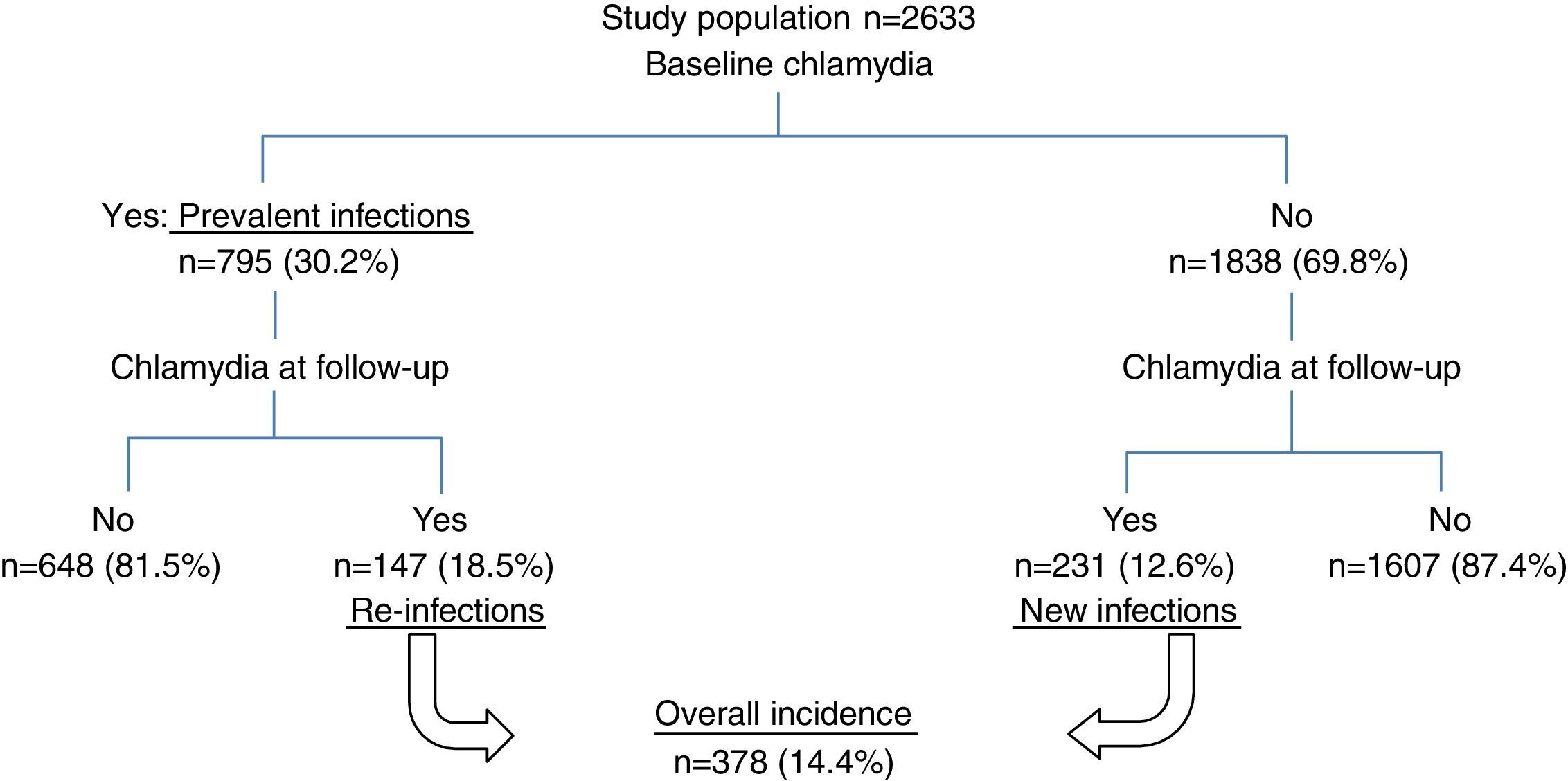
We defined cases diagnosed in the baseline episode as prevalent infections and cases diagnosed during the follow-up period as incident infections. Incident infections were classified as re-infections when they occurred in patients with positive C. trachomatis DNA tests at baseline or as new infections when they occurred in patients with negative tests at baseline. To describe prevalence, we used information from the baseline episode and to determine the factors associated with incidence, we used the information collected when the incident infection occurred.
We analyzed the following variables: age, sex, country of origin (Spain, other), sexual orientation, presence of a stable partner, number of sexual partners in the previous month and year, condom use (always/sometimes or never), whether they were working as a sex worker, history of STIs and co-infection with HIV.
In the descriptive analysis, we used measures of central tendency, t tests and chi-square tests. We calculated the cumulative incidence with 95% confidence intervals (CI) and incidence rates, expressed as the number of cases per 100 person-years of follow-up. These rates were compared between subgroups by estimating the incidence rate ratios and their 95% CI with univariate Poisson regression analysis. Kaplan–Meier survival analysis was used to estimate the cumulative incidence risk of C. trachomatis over time.
For multivariate analysis, Cox regression analysis was performed with the time between inclusion in the study and the diagnosis of C. trachomatis infection or the last negative test result as the dependent variable. When the effect of any of the explanatory variables, such as having been infected with C. trachomatis at baseline, was found to be time dependent, Heaviside functions were included in the models to estimate the hazard ratios (HRs) in the different follow-up periods.
The statistical analysis was carried out using the SAS statistical software v 9.2 (SAS Institute, Cary, NC, USA).
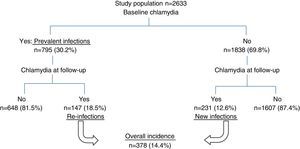
ResultsA total of 2633 patients met the selection criteria for the study. Of these, 795 patients (30.2%, 95% CI: 28.4–31.9) tested positive for C. trachomatis in the baseline episode (prevalence of chlamydia) and all had been treated with azithromycin or doxycycline.
The median period of follow-up was 13 months (0.9–101.4 months). Overall, 59% of participants were male, of whom 36.7% were MSM. The mean age on inclusion was 32.5 years (14–80 years), men being older than women (33.6 vs 31.0 years old, p<.0001). Regarding place of origin, 41.5% were immigrants, mainly from Latin America and Africa. Of the total, 13.6% were sex workers (97% women). The prevalence of HIV infection was 5.7% (7.7% among men vs 2.8% among women, p<0.0001), and a further 42 patients who tested negative for HIV at baseline became infected with the virus during the follow-up period.
Fig. 1 shows the flow of patients through the study.
Overall incidenceA total of 378 patients (14.4%) had at least one C. trachomatis infection during the study period, with an incidence rate of 7.97 cases per 100 person-years (95% CI: 7.2–8.8). The rate was higher at 43.3 per 100 person-years (95% CI: 26.5–70.6) in the under-20-year-olds, with a cumulative incidence of 26.7% (95% CI: 15.5–37.9).
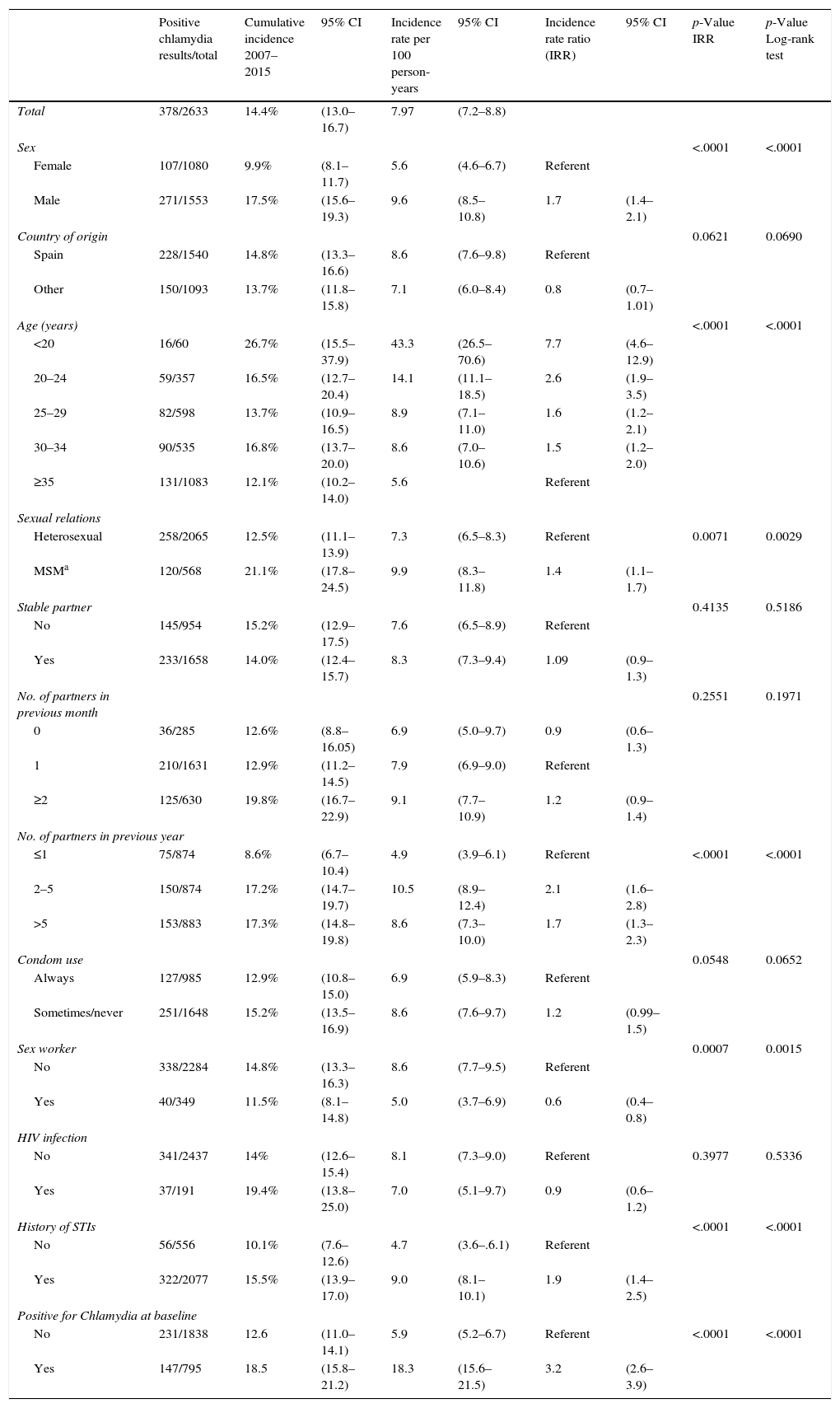
Table 1 summarizes the variables associated with total incidence of C. trachomatis in the univariate analysis: sex (men), age, sexual orientation (MSM), having had more than one partner in the previous year, having a history of STIs, having been diagnosed with chlamydia at baseline, and being a sex worker. The last variable, unlike the others, was negatively associated with incidence: incidence rate ratio=0.6 (95% CI: 0.4–0.8).
Overall incidence of C. trachomatis. Univariate analysis.
| Positive chlamydia results/total | Cumulative incidence 2007–2015 | 95% CI | Incidence rate per 100 person-years | 95% CI | Incidence rate ratio (IRR) | 95% CI | p-Value IRR | p-Value Log-rank test | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 378/2633 | 14.4% | (13.0–16.7) | 7.97 | (7.2–8.8) | ||||
| Sex | <.0001 | <.0001 | |||||||
| Female | 107/1080 | 9.9% | (8.1–11.7) | 5.6 | (4.6–6.7) | Referent | |||
| Male | 271/1553 | 17.5% | (15.6–19.3) | 9.6 | (8.5–10.8) | 1.7 | (1.4–2.1) | ||
| Country of origin | 0.0621 | 0.0690 | |||||||
| Spain | 228/1540 | 14.8% | (13.3–16.6) | 8.6 | (7.6–9.8) | Referent | |||
| Other | 150/1093 | 13.7% | (11.8–15.8) | 7.1 | (6.0–8.4) | 0.8 | (0.7–1.01) | ||
| Age (years) | <.0001 | <.0001 | |||||||
| <20 | 16/60 | 26.7% | (15.5–37.9) | 43.3 | (26.5–70.6) | 7.7 | (4.6–12.9) | ||
| 20–24 | 59/357 | 16.5% | (12.7–20.4) | 14.1 | (11.1–18.5) | 2.6 | (1.9–3.5) | ||
| 25–29 | 82/598 | 13.7% | (10.9–16.5) | 8.9 | (7.1–11.0) | 1.6 | (1.2–2.1) | ||
| 30–34 | 90/535 | 16.8% | (13.7–20.0) | 8.6 | (7.0–10.6) | 1.5 | (1.2–2.0) | ||
| ≥35 | 131/1083 | 12.1% | (10.2–14.0) | 5.6 | Referent | ||||
| Sexual relations | |||||||||
| Heterosexual | 258/2065 | 12.5% | (11.1–13.9) | 7.3 | (6.5–8.3) | Referent | 0.0071 | 0.0029 | |
| MSMa | 120/568 | 21.1% | (17.8–24.5) | 9.9 | (8.3–11.8) | 1.4 | (1.1–1.7) | ||
| Stable partner | 0.4135 | 0.5186 | |||||||
| No | 145/954 | 15.2% | (12.9–17.5) | 7.6 | (6.5–8.9) | Referent | |||
| Yes | 233/1658 | 14.0% | (12.4–15.7) | 8.3 | (7.3–9.4) | 1.09 | (0.9–1.3) | ||
| No. of partners in previous month | 0.2551 | 0.1971 | |||||||
| 0 | 36/285 | 12.6% | (8.8–16.05) | 6.9 | (5.0–9.7) | 0.9 | (0.6–1.3) | ||
| 1 | 210/1631 | 12.9% | (11.2–14.5) | 7.9 | (6.9–9.0) | Referent | |||
| ≥2 | 125/630 | 19.8% | (16.7–22.9) | 9.1 | (7.7–10.9) | 1.2 | (0.9–1.4) | ||
| No. of partners in previous year | |||||||||
| ≤1 | 75/874 | 8.6% | (6.7–10.4) | 4.9 | (3.9–6.1) | Referent | <.0001 | <.0001 | |
| 2–5 | 150/874 | 17.2% | (14.7–19.7) | 10.5 | (8.9–12.4) | 2.1 | (1.6–2.8) | ||
| >5 | 153/883 | 17.3% | (14.8–19.8) | 8.6 | (7.3–10.0) | 1.7 | (1.3–2.3) | ||
| Condom use | 0.0548 | 0.0652 | |||||||
| Always | 127/985 | 12.9% | (10.8–15.0) | 6.9 | (5.9–8.3) | Referent | |||
| Sometimes/never | 251/1648 | 15.2% | (13.5–16.9) | 8.6 | (7.6–9.7) | 1.2 | (0.99–1.5) | ||
| Sex worker | 0.0007 | 0.0015 | |||||||
| No | 338/2284 | 14.8% | (13.3–16.3) | 8.6 | (7.7–9.5) | Referent | |||
| Yes | 40/349 | 11.5% | (8.1–14.8) | 5.0 | (3.7–6.9) | 0.6 | (0.4–0.8) | ||
| HIV infection | |||||||||
| No | 341/2437 | 14% | (12.6–15.4) | 8.1 | (7.3–9.0) | Referent | 0.3977 | 0.5336 | |
| Yes | 37/191 | 19.4% | (13.8–25.0) | 7.0 | (5.1–9.7) | 0.9 | (0.6–1.2) | ||
| History of STIs | <.0001 | <.0001 | |||||||
| No | 56/556 | 10.1% | (7.6–12.6) | 4.7 | (3.6–.6.1) | Referent | |||
| Yes | 322/2077 | 15.5% | (13.9–17.0) | 9.0 | (8.1–10.1) | 1.9 | (1.4–2.5) | ||
| Positive for Chlamydia at baseline | |||||||||
| No | 231/1838 | 12.6 | (11.0–14.1) | 5.9 | (5.2–6.7) | Referent | <.0001 | <.0001 | |
| Yes | 147/795 | 18.5 | (15.8–21.2) | 18.3 | (15.6–21.5) | 3.2 | (2.6–3.9) | ||
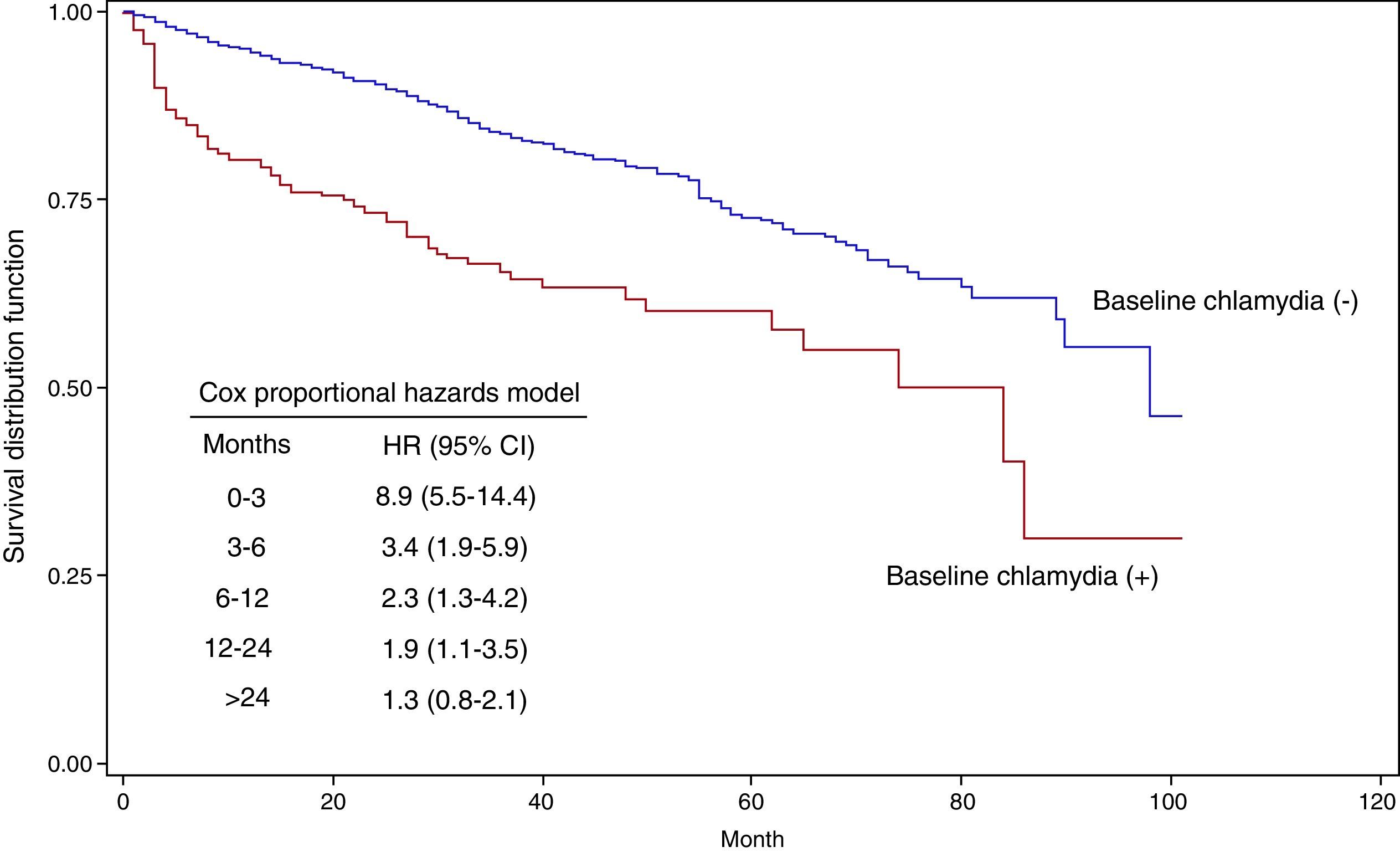
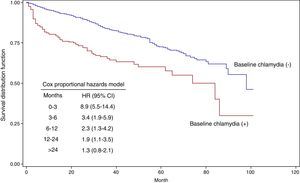
Fig. 2 shows the Kaplan–Meier survival curves as a function of having had a positive C. trachomatis test in the baseline episode. The HR for those with a positive test was 8.9 (95% CI: 5.5–14.4) in the first 3 months of follow-up, 3.4 (95% CI: 1.9–5.9) between month 3 and 6, 2.3 (95% CI: 1.3–4.2) between month 6 and 12, and 1.9 (95% CI: 1.1–3.5) between month 12 and 24. From month 24, the HR was not different from 1.
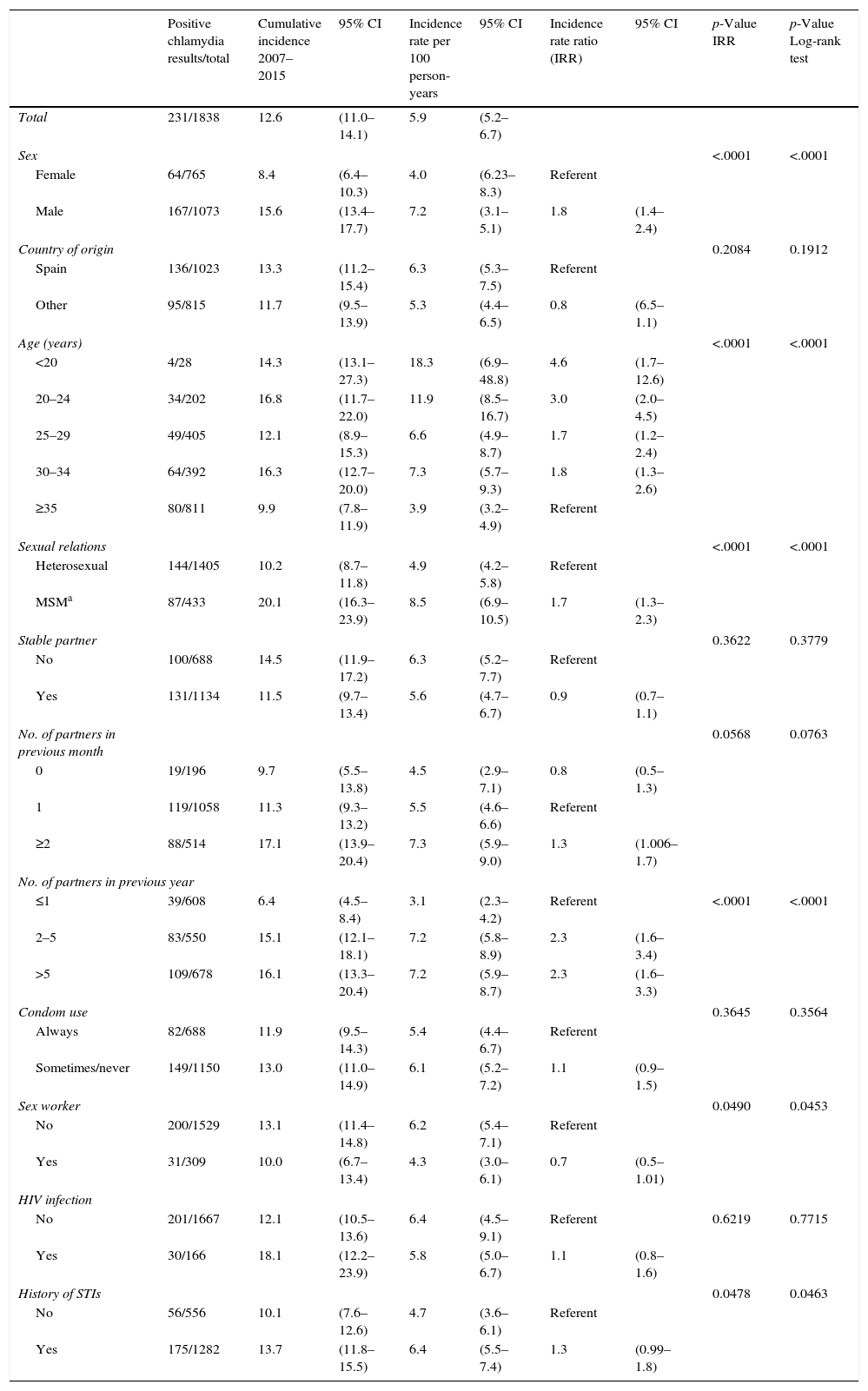
New infectionsOut of the 1838 chlamydia-free patients in the baseline episode, 231 (12.6%) had a chlamydial infection at some point during the study period, yielding an incidence rate of 5.9 per 100 person-years. By age, the highest rate of 18.3 per 100 person-years (95% CI: 6.9–48.8) was found in under-20-year-olds. The univariate analysis indicated the same associations as for total incidence (Table 2).
New infections. Univariate analysis.
| Positive chlamydia results/total | Cumulative incidence 2007–2015 | 95% CI | Incidence rate per 100 person-years | 95% CI | Incidence rate ratio (IRR) | 95% CI | p-Value IRR | p-Value Log-rank test | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 231/1838 | 12.6 | (11.0–14.1) | 5.9 | (5.2–6.7) | ||||
| Sex | <.0001 | <.0001 | |||||||
| Female | 64/765 | 8.4 | (6.4–10.3) | 4.0 | (6.23–8.3) | Referent | |||
| Male | 167/1073 | 15.6 | (13.4–17.7) | 7.2 | (3.1–5.1) | 1.8 | (1.4–2.4) | ||
| Country of origin | 0.2084 | 0.1912 | |||||||
| Spain | 136/1023 | 13.3 | (11.2–15.4) | 6.3 | (5.3–7.5) | Referent | |||
| Other | 95/815 | 11.7 | (9.5–13.9) | 5.3 | (4.4–6.5) | 0.8 | (6.5–1.1) | ||
| Age (years) | <.0001 | <.0001 | |||||||
| <20 | 4/28 | 14.3 | (13.1–27.3) | 18.3 | (6.9–48.8) | 4.6 | (1.7–12.6) | ||
| 20–24 | 34/202 | 16.8 | (11.7–22.0) | 11.9 | (8.5–16.7) | 3.0 | (2.0–4.5) | ||
| 25–29 | 49/405 | 12.1 | (8.9–15.3) | 6.6 | (4.9–8.7) | 1.7 | (1.2–2.4) | ||
| 30–34 | 64/392 | 16.3 | (12.7–20.0) | 7.3 | (5.7–9.3) | 1.8 | (1.3–2.6) | ||
| ≥35 | 80/811 | 9.9 | (7.8–11.9) | 3.9 | (3.2–4.9) | Referent | |||
| Sexual relations | <.0001 | <.0001 | |||||||
| Heterosexual | 144/1405 | 10.2 | (8.7–11.8) | 4.9 | (4.2–5.8) | Referent | |||
| MSMa | 87/433 | 20.1 | (16.3–23.9) | 8.5 | (6.9–10.5) | 1.7 | (1.3–2.3) | ||
| Stable partner | 0.3622 | 0.3779 | |||||||
| No | 100/688 | 14.5 | (11.9–17.2) | 6.3 | (5.2–7.7) | Referent | |||
| Yes | 131/1134 | 11.5 | (9.7–13.4) | 5.6 | (4.7–6.7) | 0.9 | (0.7–1.1) | ||
| No. of partners in previous month | 0.0568 | 0.0763 | |||||||
| 0 | 19/196 | 9.7 | (5.5–13.8) | 4.5 | (2.9–7.1) | 0.8 | (0.5–1.3) | ||
| 1 | 119/1058 | 11.3 | (9.3–13.2) | 5.5 | (4.6–6.6) | Referent | |||
| ≥2 | 88/514 | 17.1 | (13.9–20.4) | 7.3 | (5.9–9.0) | 1.3 | (1.006–1.7) | ||
| No. of partners in previous year | |||||||||
| ≤1 | 39/608 | 6.4 | (4.5–8.4) | 3.1 | (2.3–4.2) | Referent | <.0001 | <.0001 | |
| 2–5 | 83/550 | 15.1 | (12.1–18.1) | 7.2 | (5.8–8.9) | 2.3 | (1.6–3.4) | ||
| >5 | 109/678 | 16.1 | (13.3–20.4) | 7.2 | (5.9–8.7) | 2.3 | (1.6–3.3) | ||
| Condom use | 0.3645 | 0.3564 | |||||||
| Always | 82/688 | 11.9 | (9.5–14.3) | 5.4 | (4.4–6.7) | Referent | |||
| Sometimes/never | 149/1150 | 13.0 | (11.0–14.9) | 6.1 | (5.2–7.2) | 1.1 | (0.9–1.5) | ||
| Sex worker | 0.0490 | 0.0453 | |||||||
| No | 200/1529 | 13.1 | (11.4–14.8) | 6.2 | (5.4–7.1) | Referent | |||
| Yes | 31/309 | 10.0 | (6.7–13.4) | 4.3 | (3.0–6.1) | 0.7 | (0.5–1.01) | ||
| HIV infection | |||||||||
| No | 201/1667 | 12.1 | (10.5–13.6) | 6.4 | (4.5–9.1) | Referent | 0.6219 | 0.7715 | |
| Yes | 30/166 | 18.1 | (12.2–23.9) | 5.8 | (5.0–6.7) | 1.1 | (0.8–1.6) | ||
| History of STIs | 0.0478 | 0.0463 | |||||||
| No | 56/556 | 10.1 | (7.6–12.6) | 4.7 | (3.6–6.1) | Referent | |||
| Yes | 175/1282 | 13.7 | (11.8–15.5) | 6.4 | (5.5–7.4) | 1.3 | (0.99–1.8) | ||
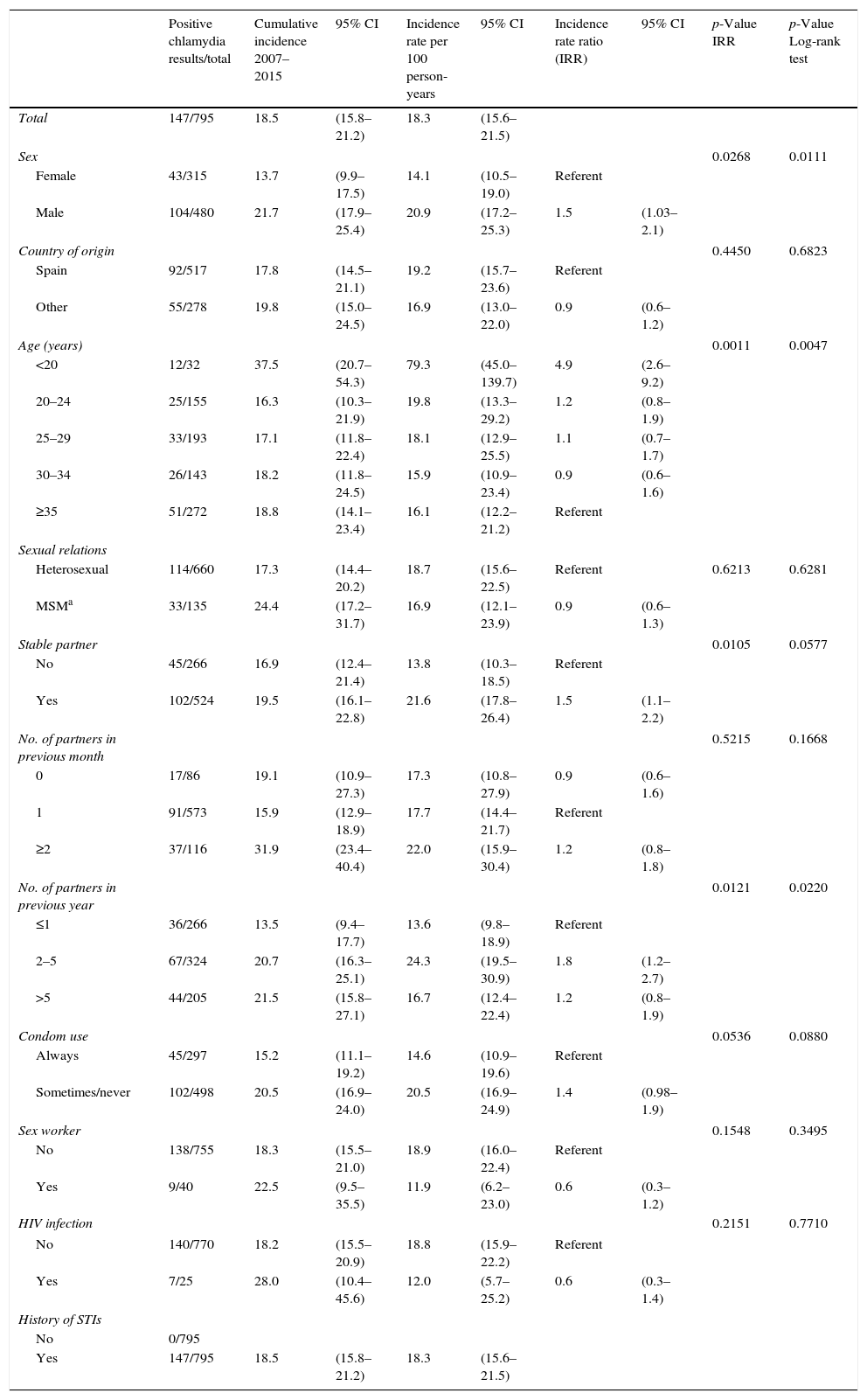
Overall, 18.5% (147) of the 795 patients with a positive C. trachomatis test in the baseline episode had a re-infection, with a rate of 18.3 per 100 person-years. The rate of re-infections among those under 20 years old was 79.3 per 100 person-years (95% CI: 45–139.7). Table 3 summarizes the cumulative incidence and re-infection rates of the different patient groups, as well as the incidence rate ratio of the factors associated with C. trachomatis re-infection in the univariate analysis: sex, age, having a stable partner and number of partners in the previous year.
Re-infections. Univariate analysis.
| Positive chlamydia results/total | Cumulative incidence 2007–2015 | 95% CI | Incidence rate per 100 person-years | 95% CI | Incidence rate ratio (IRR) | 95% CI | p-Value IRR | p-Value Log-rank test | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 147/795 | 18.5 | (15.8–21.2) | 18.3 | (15.6–21.5) | ||||
| Sex | 0.0268 | 0.0111 | |||||||
| Female | 43/315 | 13.7 | (9.9–17.5) | 14.1 | (10.5–19.0) | Referent | |||
| Male | 104/480 | 21.7 | (17.9–25.4) | 20.9 | (17.2–25.3) | 1.5 | (1.03–2.1) | ||
| Country of origin | 0.4450 | 0.6823 | |||||||
| Spain | 92/517 | 17.8 | (14.5–21.1) | 19.2 | (15.7–23.6) | Referent | |||
| Other | 55/278 | 19.8 | (15.0–24.5) | 16.9 | (13.0–22.0) | 0.9 | (0.6–1.2) | ||
| Age (years) | 0.0011 | 0.0047 | |||||||
| <20 | 12/32 | 37.5 | (20.7–54.3) | 79.3 | (45.0–139.7) | 4.9 | (2.6–9.2) | ||
| 20–24 | 25/155 | 16.3 | (10.3–21.9) | 19.8 | (13.3–29.2) | 1.2 | (0.8–1.9) | ||
| 25–29 | 33/193 | 17.1 | (11.8–22.4) | 18.1 | (12.9–25.5) | 1.1 | (0.7–1.7) | ||
| 30–34 | 26/143 | 18.2 | (11.8–24.5) | 15.9 | (10.9–23.4) | 0.9 | (0.6–1.6) | ||
| ≥35 | 51/272 | 18.8 | (14.1–23.4) | 16.1 | (12.2–21.2) | Referent | |||
| Sexual relations | |||||||||
| Heterosexual | 114/660 | 17.3 | (14.4–20.2) | 18.7 | (15.6–22.5) | Referent | 0.6213 | 0.6281 | |
| MSMa | 33/135 | 24.4 | (17.2–31.7) | 16.9 | (12.1–23.9) | 0.9 | (0.6–1.3) | ||
| Stable partner | 0.0105 | 0.0577 | |||||||
| No | 45/266 | 16.9 | (12.4–21.4) | 13.8 | (10.3–18.5) | Referent | |||
| Yes | 102/524 | 19.5 | (16.1–22.8) | 21.6 | (17.8–26.4) | 1.5 | (1.1–2.2) | ||
| No. of partners in previous month | 0.5215 | 0.1668 | |||||||
| 0 | 17/86 | 19.1 | (10.9–27.3) | 17.3 | (10.8–27.9) | 0.9 | (0.6–1.6) | ||
| 1 | 91/573 | 15.9 | (12.9–18.9) | 17.7 | (14.4–21.7) | Referent | |||
| ≥2 | 37/116 | 31.9 | (23.4–40.4) | 22.0 | (15.9–30.4) | 1.2 | (0.8–1.8) | ||
| No. of partners in previous year | 0.0121 | 0.0220 | |||||||
| ≤1 | 36/266 | 13.5 | (9.4–17.7) | 13.6 | (9.8–18.9) | Referent | |||
| 2–5 | 67/324 | 20.7 | (16.3–25.1) | 24.3 | (19.5–30.9) | 1.8 | (1.2–2.7) | ||
| >5 | 44/205 | 21.5 | (15.8–27.1) | 16.7 | (12.4–22.4) | 1.2 | (0.8–1.9) | ||
| Condom use | 0.0536 | 0.0880 | |||||||
| Always | 45/297 | 15.2 | (11.1–19.2) | 14.6 | (10.9–19.6) | Referent | |||
| Sometimes/never | 102/498 | 20.5 | (16.9–24.0) | 20.5 | (16.9–24.9) | 1.4 | (0.98–1.9) | ||
| Sex worker | 0.1548 | 0.3495 | |||||||
| No | 138/755 | 18.3 | (15.5–21.0) | 18.9 | (16.0–22.4) | Referent | |||
| Yes | 9/40 | 22.5 | (9.5–35.5) | 11.9 | (6.2–23.0) | 0.6 | (0.3–1.2) | ||
| HIV infection | 0.2151 | 0.7710 | |||||||
| No | 140/770 | 18.2 | (15.5–20.9) | 18.8 | (15.9–22.2) | Referent | |||
| Yes | 7/25 | 28.0 | (10.4–45.6) | 12.0 | (5.7–25.2) | 0.6 | (0.3–1.4) | ||
| History of STIs | |||||||||
| No | 0/795 | ||||||||
| Yes | 147/795 | 18.5 | (15.8–21.2) | 18.3 | (15.6–21.5) | ||||
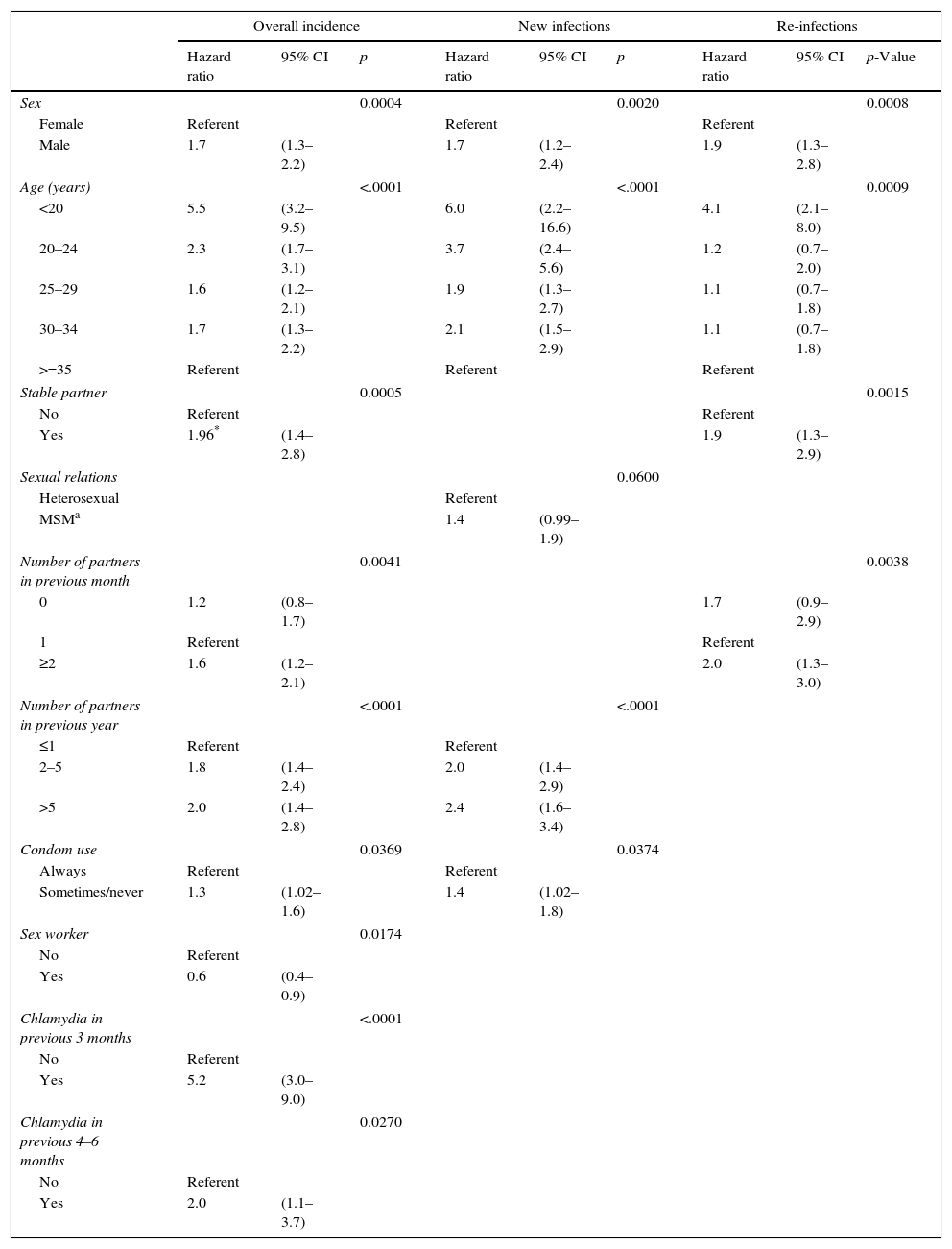
In the analysis using the Cox proportional hazards model, the factors most closely associated with the overall incidence of C. trachomatis were a recent previous chlamydial infection and young age. A history of chlamydial infection in the previous 3 months or between 4 and 6 months previously was associated with HRs of 5.2 (95% CI: 3.6–9.0) and 2.0 (95% CI: 1.1–3.7) respectively, while for infections more than 6 months earlier, the HR was not significantly different from 1. Compared to patients aged 35 years old or above, the risk was significantly greater in younger groups, especially those under 20 years of age: HR 5.5 (95% CI: 3.2–9.5).
Other variables independently associated with C. trachomatis incidence were being male, having had two or more partners in the previous month and year, using condoms inconsistently and being a sex worker, which continued to have a similar negative association to that observed in the univariate analysis. Having a stable partner was only associated with a greater incidence risk of C. trachomatis in those who had had chlamydial infection in the baseline episode (Table 4).
Factors associated with overall incidence of C. trachomatis, new infections and re-infections. Multivariate analysis (Cox regression analysis).
| Overall incidence | New infections | Re-infections | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p-Value | |
| Sex | 0.0004 | 0.0020 | 0.0008 | ||||||
| Female | Referent | Referent | Referent | ||||||
| Male | 1.7 | (1.3–2.2) | 1.7 | (1.2–2.4) | 1.9 | (1.3–2.8) | |||
| Age (years) | <.0001 | <.0001 | 0.0009 | ||||||
| <20 | 5.5 | (3.2–9.5) | 6.0 | (2.2–16.6) | 4.1 | (2.1–8.0) | |||
| 20–24 | 2.3 | (1.7–3.1) | 3.7 | (2.4–5.6) | 1.2 | (0.7–2.0) | |||
| 25–29 | 1.6 | (1.2–2.1) | 1.9 | (1.3–2.7) | 1.1 | (0.7–1.8) | |||
| 30–34 | 1.7 | (1.3–2.2) | 2.1 | (1.5–2.9) | 1.1 | (0.7–1.8) | |||
| >=35 | Referent | Referent | Referent | ||||||
| Stable partner | 0.0005 | 0.0015 | |||||||
| No | Referent | Referent | |||||||
| Yes | 1.96* | (1.4–2.8) | 1.9 | (1.3–2.9) | |||||
| Sexual relations | 0.0600 | ||||||||
| Heterosexual | Referent | ||||||||
| MSMa | 1.4 | (0.99–1.9) | |||||||
| Number of partners in previous month | 0.0041 | 0.0038 | |||||||
| 0 | 1.2 | (0.8–1.7) | 1.7 | (0.9–2.9) | |||||
| 1 | Referent | Referent | |||||||
| ≥2 | 1.6 | (1.2–2.1) | 2.0 | (1.3–3.0) | |||||
| Number of partners in previous year | <.0001 | <.0001 | |||||||
| ≤1 | Referent | Referent | |||||||
| 2–5 | 1.8 | (1.4–2.4) | 2.0 | (1.4–2.9) | |||||
| >5 | 2.0 | (1.4–2.8) | 2.4 | (1.6–3.4) | |||||
| Condom use | 0.0369 | 0.0374 | |||||||
| Always | Referent | Referent | |||||||
| Sometimes/never | 1.3 | (1.02–1.6) | 1.4 | (1.02–1.8) | |||||
| Sex worker | 0.0174 | ||||||||
| No | Referent | ||||||||
| Yes | 0.6 | (0.4–0.9) | |||||||
| Chlamydia in previous 3 months | <.0001 | ||||||||
| No | Referent | ||||||||
| Yes | 5.2 | (3.0–9.0) | |||||||
| Chlamydia in previous 4–6 months | 0.0270 | ||||||||
| No | Referent | ||||||||
| Yes | 2.0 | (1.1–3.7) | |||||||
Considering new infections alone, the factors that were independently associated with a higher incidence of C. trachomatis were: male sex (HR=1.7; 95% CI: 1.2–2.4), younger age (HR=6.0 for patients under 20 years old compared to those over 35), more partners in the previous year (HR=2.0 for those reporting 2–5 partners and 2.4 for those reporting more than 5 partners, compared to those with 1 partner) and inconsistent condom use (HR=1.4; 95% CI: 1.02–1.8). The type of sexual relations was close to statistical significance, with a HR of 1.4 for MSM (95% CI: 0.99–1.9; p=0.060) (Table 4).
In the multivariate analysis, being male, being in the youngest age group, and having a stable partner continued to be associated with chlamydia re-infection: HR=1.9 (95% CI: 1.3–2.8) for males; HR=4.1 (95% CI: 2.1–8.0) for under-20-year-olds vs over-35-year-olds; and HR=1.9 (95% CI: 1.3–2.9) for those with a stable partner. The number of partners in the previous year was not associated with reinfections in the multivariate analysis. We did, however, find an association between the number of partners in the previous month and re-infections, with a two-fold higher risk of re-infection among those with two or more partners (HR=2.0; 95% CI: 1.3–3.0) (Table 4).
DiscussionTo our knowledge, this is the first study undertaken in Spain with a large sample of patients of both sexes and broad range of age, to estimate the incidence of C. trachomatis and assess differences between the factors associated with new infections and re-infections.
We have found an overall incidence rate of 8 cases per 100 person-years seen in an STD clinic, with an incidence of new infections of 6 cases per 100 person-years and a high rate of re-infections: 18.3 per 100 person-years (rising to 79.3 per 100 person-years among under-20-year-olds). These rates are lower than those observed in other studies in patients from STI clinics, with rates of re-infection of between 21 and 39 cases per 100 person-years.18–20
As in previous studies,18,21–23 the risk of C. trachomatis infection is strongly associated with having previously been infected with this microorganism. Our results show that, independent of the other factors studied, the risk of re-infection is five-fold higher than that of a new infection considering the 3 months following an episode and two-fold higher considering the period from 4 to 6 months. After 6 months, we did not find differences in the risk of infection between those with positive and negative baseline test results.
Stratifying data to new infections or re-infections, younger age and male sex were found to be predictive factors. More partners in the previous year and inconsistent condom use were associated with new infections, while having a stable partner and more partners in the previous month were associated with re-infection.
The risk of re-infection is highest in the first months, and hence, it is logical that this factor is associated with the number of partners in the previous month, and it is known that it is strongly associated with having untreated partners.24 Our only way of notifying contacts is patient referral and we had only treated at least one contact in the case of 59% of patients positive for C. trachomatis at baseline. This would explain the association between re-infection and having a stable partner, as this partner had probably not been treated.
Although most studies on C. trachomatis are focused on women, in research considering infection in both sexes, the rates of C. trachomatis re-infection among men and women are comparable.21 In contrast, in our study, the incidence rates have been significantly higher in men.
Our study has several limitations that should be borne in mind when interpreting the results. First, the study was based on a sample of patients seeking medical attention in an STI clinic, and hence, the results cannot be applied to the general population. For example, the paradoxical finding that being a sex worker is associated with a lower likelihood of being infected with C. trachomatis may be explained by the selection bias of this study in terms of the different rates of participation of two subsets of sex workers: those who attend medical check-ups and those who do not. Those who attend medical check-ups, come to our clinic on a regular basis for STI testing, and it is likely that these individuals take greater care of their own health and more preventative measures than other sex workers who do not attend check-ups, and who are underrepresented in this study.
Second, this is a retrospective study of a cohort of people who have undergone C. trachomatis tests at least twice. This may influence the estimation of incidence, since the reasons why someone comes back seeking a new medical consultation may not be independent of the risk of having a STI, and hence high-risk patients may come back earlier than their low-risk counterparts. This bias has a greater impact on studies with short periods of follow-up, and may lead to an overestimation of the incidence.25 In our study, the median follow-up period was 13 months (4.3 months in patients with a positive C. trachomatis result at baseline and 18.6 months in those with negative results).
Third, our standardized clinical encounter form collects little information on the number and type of contacts treated, hindering analysis of this variable. Finally, though the rate of failure using azithromycin and doxycycline are low, we cannot rule out the occurrence of some cases of therapeutic failure.
Though the study has limitations which may restrict the generalization of results, these are similar to those reported in other population-based studies,21,22,26 and although our study was focused on a sample of patients from an STI clinic, this clinic has a defined population catchment area.
The provision of care for STIs varies across Spain and there is no organized chlamydia control activity. Our results indicate that the risk of infection with C. trachomatis is strongly associated with having a history of this type of infection, and that this risk is independent of patient age. Therefore, treating the infection is not enough. We must establish guidelines for chlamydia in Spain that go beyond establishing the antibiotic treatment of choice, to also include recommendations on the need for follow-up and repeat testing, independently of age. These patients should also participate in additional interventions such as active recall to increase re-testing rates.
Data about the optimal timing of repeated testing are inconclusive and it is recommended that each country conduct their own research to inform local guidelines.27 Mathematical models have found that the optimal re-testing interval is between 2 and 5 months after the initial test28 and the majority of countries with recommendations on this matter do it between 3 and 6 months.15–17,29,30 Our results support the view that this re-testing interval should be used in our setting.
It is also necessary to establish more effective mechanisms for facilitating the notification and management of sexual partners, this currently being limited to patient referral.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingCall for Research Grants 2015 Integrated Health Organization Bilbao-Basurto (Osakidetza).