Current guidelines for the microbiological diagnosis of ventilator-associated pneumonia (VAP) are imprecise. Based on data provided by intensive care specialists (ICS) and microbiologists, this study defines the clinical practices and microbiological techniques currently used for an aetiological diagnosis of VAP and pinpoints deficiencies.
MethodsEighty hospitals in the national health network with intensive care and microbiology departments were sent two questionnaires, one for each department, in order to collect data on VAP diagnosis for the previous year.
ResultsOut of the 80 hospitals, 35 (43.8%) hospitals participated. These included 673 ICU beds, 32,020 ICU admissions, 173,820 ICU days stay, and generated 27,048 lower respiratory tract specimens in the year. A third of the hospitals (35%) had a microbiology department available 24/7. Most samples (83%) were tracheal aspirates. Gram stain results were immediately reported in around half (47%) of the hospitals. Quantification was made in 75% of hospitals. Molecular techniques and direct susceptibility testing were performed in 12% and one institution, respectively. Mean turnaround time for a microbiological report was 1.7 (SD; 0.7), and 2.2 (SD; 0.6) days for a negative and positive result, respectively. Telephone/in-person information was offered by 65% of the hospitals. Most (89%) ICS considered microbiological information as very useful. No written procedures were available in half the ICUs.
ConclusionsBoth ICS and microbiologists agreed that present guidelines for the diagnosis of VAP could be much improved, and that a new set of consensus guidelines is urgently required. A need for guidelines to be more effectively implemented was also identified in order to improve outcomes in patients with VAP.
Las guías para el diagnóstico microbiológico de la neumonía asociada a la ventilación mecánica (NAVM) son imprecisas. Este estudio describe la práctica clínica y las técnicas microbiológicas según la información proporcionada por intensivistas y microbiólogos de varias UCIs españolas, e identifica deficiencias y oportunidades de mejora.
MétodosSe enviaron cuestionarios a 80 hospitales públicos españoles con servicios de cuidados intensivos y de microbiología, solicitando datos sobre el diagnóstico de la NAVM en el año anterior.
ResultadosLos 35 hospitales participantes (43,8%) abarcaron 673 camas de UCI con 32.020 admisiones y 173,820 estancias, y generaron 27.048 muestras del tracto respiratorio inferior. El 35% disponía de un servicio de microbiología 24/7. El 83% de las muestras fueron aspirados endotraqueales; el 47% de los Gram se informaron de inmediato y el 75% de las muestras se procesaron de manera semicuantitativa. Las técnicas moleculares y el antibiograma directo se realizaron en el 12% de los casos y en una institución, respectivamente. La respuesta media fue de 1,7 (DE 0,7) y 2,2 (SD 0,6) días para un cultivo negativo y uno positivo, respectivamente. La información directa estuvo disponible en el 65% de los hospitales. El 89% de los intensivistas consideraron muy útil la información microbiológica. En la mitad de la UCIs no había protocolos escritos de manejo de esta entidad.
ConclusionesIntensivistas y microbiólogos coinciden en que las guías actuales para el diagnóstico de NAVM se podrían mejorar significativamente. También es necesario implementar las guías de manera más efectiva para mejorar la evolución de los pacientes.
Hospital-acquired pneumonia, particularly ventilator-associated pneumonia (VAP), is one of the leading causes of infection and death in the healthcare setting.1 VAP is associated with prolonged hospitalization and increased health care costs.2 The incorrect or delayed treatment of VAP in the first few hours gives rise to a worse prognosis.3 VAP should thus be considered a microbiological emergency, yet in many institutions a microbiological diagnosis of VAP is not available around the clock. Information from the microbiology department serves to shorten the period of broad-spectrum empirical treatment and thus ensure a more restricted use of antimicrobial agents.4
Current VAP guidelines are imprecise about many issues related to its microbiological diagnosis5–8 such as the most suitable type of specimen, sample preservation, transport to the laboratory, attention given to these samples or results interpretation.
In addition, there is little information on present microbiological practices and their relative capacity to diagnose VAP.9,10 Individual studies suggest that practice often differs very much from literature recommendations.
The present study surveys several issues regarding the microbiological diagnosis of VAP in ICUs in Spanish hospitals from the perspective of both intensivists and microbiologists. The data collected in questionnaires were used to better define the approach taken in clinical practice and to pinpoint deficiencies that will help improve current guidelines on this important topic.
MethodsStudy designIn this multicenter, multidisciplinary survey, 80 hospitals in the national health network with intensive care and microbiology departments were invited to participate via At each center accepting the invitation, intensivists and microbiologists completed a questionnaire regarding their standard approach to the management of patients with VAP.
Data collectedParticipating clinicians were asked to report data collected during the previous year about their respective hospitals and units, including hospital size, number of patients admitted, and other questions (see Tables 1–4).
Microbiological data. Quantitative variables.
| Item | Valid number | Median | P25 | P75 | Sum |
|---|---|---|---|---|---|
| Number of beds | 34 | 577 | 300.8 | 864.8 | 21524 |
| Admissions in the previous year | 33 | 20,603 | 13,119 | 35,143 | 857,631 |
| Stays in the previous year | 32 | 156,816 | 80,903 | 265,641.3 | 5,432,443 |
| Population attended (inhabitants) | 34 | 259,967.5 | 141,107 | 422,221 | 10,033,066 |
| Number of microbiologists | 34 | 5 | 2.8 | 8.5 | 214 |
| Number of residents/year | 34 | 0.5 | 0 | 1 | 24 |
| Laboratory technicians | 33 | 10 | 4.5 | 21 | 413 |
| Microbiology samples (not including blood) in the previous year | 34 | 65,791.5 | 37,114.8 | 112,908.3 | 2,581,112 |
| Lower respiratory tract samples in the previous year | 34 | 4379 | 1884.3 | 6867.5 | 176,845 |
| Respiratory samples from adult ICUs in the previous year | 34 | 749 | 299.5 | 1077.3 | 27,048 |
| Patients corresponding to these samples | 21 | 308 | 195 | 552 | 8385 |
| Tracheobronchial aspirate | 34 | 597.5 | 246 | 947.8 | 22,426 |
| Bronchoalveolar lavage | 34 | 20 | 0 | 40 | 1637 |
| Mini bronchoalveolar lavage (blind) | 34 | 0 | 0 | 5.8 | 930 |
| Protected brush specimen | 34 | 0 | 0 | 8 | 423 |
| Blind protected brush specimen | 34 | 0 | 0 | 0 | 282 |
| Transthoracic biopsy | 34 | 0 | 0 | 0 | 73 |
| Open lung biopsy | 34 | 0 | 0 | 2 | 93 |
| Pleural fluids | 34 | 26.5 | 5.8 | 50.5 | 1184 |
| Turnaround time (TAT) for a negative culture (days) | 34 | 2 | 1 | 2 | 59 |
| Increase in TAT for a negative culture because of a weekend (days) | 34 | 2 | 1 | 2 | 53 |
| TAT for a positive culture (days) | 34 | 2 | 2 | 3 | 76 |
| Increase in TAT for a positive culture because of a weekend (days) | 34 | 2 | 1 | 2 | 52 |
| Incubation period for fungal plates (days) | 33 | 10 | 4.5 | 21 | 413 |
| Perceived quality of microbiological diagnosis of VAP | 34 | 7 | 7 | 8 | 246 |
| Perceived clinical impact of microbiological diagnosis of VAP in ICU patients | 34 | 8 | 7 | 8 | 256 |
| Perceived ICU rating of microbiological diagnosis of VAP | 34 | 8 | 6 | 8 | 250 |
| Factors hindering a microbiological diagnosis of VAP at your institution (0=no impact, 10=very high impact) | |||||
| • Lack of staff/technicians | 34 | 7 | 2.8 | 8 | 191 |
| • Lack of microbiologists | 34 | 5 | 0 | 7 | 153 |
| • Lack of coordination between microbiology and ICUs | 34 | 2 | 0 | 6 | 110 |
| • Lack of coordination within Microbiology Dept. | 34 | 1 | 0 | 3 | 66 |
| • Lack of resources | 34 | 7 | 3 | 8 | 190 |
| • Lack of microbiology services 24/7 | 34 | 8 | 4.8 | 10 | 235 |
| • Lack of motivation | 34 | 1 | 0 | 3 | 59 |
| • Lack of standardized procedures | 34 | 1 | 0 | 2 | 64 |
| • Lack of correlation between microbiological findings and clinical status | 33 | 3 | 1 | 5 | 108 |
| • TAT too long | 34 | 5 | 2 | 7 | 152 |
| • The clinician has difficulty in result interpretation | 34 | 3.5 | 2 | 5 | 129 |
Microbiological data. Qualitative variables.
| Item | Valid number | n | % |
|---|---|---|---|
| The microbiology laboratory at your institution is a: | 34 | ||
| • Service | 18 | 52.9 | |
| • Section | 10 | 29.4 | |
| • Unit | 4 | 11.8 | |
| • None of the above | 2 | 5.9 | |
| Processing of LRT samples at your institution can be described as: | 34 | ||
| • Immediate 7 days a week | 10 | 29.4 | |
| • Immediate 7 days a week only if requested | 5 | 14.7 | |
| • Immediate during working hours | 18 | 52.9 | |
| • No immediate processing. Batch processing | 1 | 2.9 | |
| Indicate the laboratory hours during which LRT samples are processed: | 34 | ||
| • 8:00–15:00h Mon-Fri | 4 | 11.8 | |
| • 8:00–15:00h Mon-Sat | 8 | 23.5 | |
| • 8:00–22:00 Mon-Fri | 10 | 29.4 | |
| • 8:00–15:00h plus 24-hour on-call service | 6 | 17.6 | |
| • 24-hour on-call service | 6 | 17.6 | |
| A LRT from an adult ICU receives attention from a microbiologist/resident: | 34 | ||
| • 24hours a day, 7 day per week | 4 | 11.8 | |
| • When requested | 6 | 17.6 | |
| • Only during working hours | 24 | 70.6 | |
| Microbiological results are provided as: | 34 | ||
| • Printed report | 18 | 52.9 | |
| • Printed and online report | 16 | 47.1 | |
| Preliminary microbiological reports (printed and/or online) are available for: | 34 | ||
| • Blood cultures only | 1 | 2.9 | |
| • Positive blood and CSF cultures | 3 | 8.8 | |
| • Positive blood and CSF cultures and other important patients/samples/pathogens routinely | 30 | 88.2 | |
| Telephone/in-person information is available: | 34 | ||
| • Under very specific circumstances | 1 | 2.9 | |
| • Only for blood cultures | 11 | 32.4 | |
| • Blood cultures and LRT samples from ICUs | 22 | 64.7 | |
| Indicate whether interpretation guidelines are provided with microbiological reports of LRT samples: | 34 | ||
| • The dubious pathogenic value of microorganisms such as coagulase-negative staphylococci, enterococci, etc. is usually indicated | 16 | 47.1 | |
| • Written guidelines exist for the interpretation of results | 4 | 11.8 | |
| • Occasionally a note is added to the report | 13 | 38.2 | |
| • Hardly ever | 1 | 2.9 | |
| Indicate whether preliminary susceptibility reports are provided for positive blood cultures: | 34 | ||
| • Never | 7 | 20.6 | |
| • Only if specifically requested | 2 | 5.9 | |
| • Only for important patients/pathogens selected | 11 | 32.4 | |
| • Always | 14 | 41.2 | |
| Indicate the cultures performed on LRT samples: | 34 | ||
| • Quantitative cultures (cfu/mL) | 25 | 73.5 | |
| • Semi-quantitative (cfu/plate) | 2 | 5.9 | |
| • Estimate of quantity (’+++’, significant’, etc.) | 4 | 11.8 | |
| • Not performed | 3 | 8.8 | |
| Indicate plating method for quantitative cultures: | 34 | ||
| • No quantitative cultures are done | 4 | 11.8 | |
| • Semiquantitative cultures without plating a predetermined volume | 1 | 2.9 | |
| • Calibrated loop | 20 | 58.8 | |
| • Serial dilutions plated using a pipette/micropipette | 9 | 26.5 | |
| Indicate the criteria for Gram staining of LRT samples: | 34 | ||
| • Only under specific request | 2 | 5.9 | |
| • Only for important samples | 2 | 5.9 | |
| • For all samples | 30 | 88.2 | |
| When is the Gram stain performed? | 34 | ||
| • On the same day | 25 | 73.5 | |
| • On the following day | 9 | 26.5 | |
| When is the Gram stain reported? | 34 | ||
| • It is not reported | 2 | 5.9 | |
| • Together with the culture results | 16 | 47.1 | |
| • Immediately, without waiting for culture results | 11 | 32.4 | |
| • Immediately, without waiting for culture results by telephone/in person | 5 | 14.7 | |
| Are LRT samples from patients with suspected VAP plated if the Gram stain is negative? | 34 | ||
| • Yes | 34 | 100 | |
| Indicate when/which LRT samples are processed for anaerobic bacteria: | 34 | ||
| • Hardly ever | 1 | 2.9 | |
| • Certain samples only (e.g., pleural fluid) | 18 | 52.9 | |
| • Certain samples | 12 | 35.3 | |
| • Any sample if requested | 1 | 2.9 | |
| • All LRT samples | 2 | 5.9 | |
| Is direct susceptibility testing carried out on LRT samples from patients with suspected VAP? | 34 | ||
| • No | 26 | 76.5 | |
| • Only if requested | 6 | 17.6 | |
| • Only for important samples | 1 | 2.9 | |
| • In all LRT samples from the ICU with a positive Gram stain | 1 | 2.9 | |
| Indicate the methods used for direct susceptibility testing: | 32 | ||
| • E-test, Oxoid-MICE or equivalent | 4 | 12.5 | |
| • Disk-diffusion | 5 | 15.6 | |
| • Direct susceptibility testing of LRT samples is not performed | 23 | 71.9 | |
| Is a molecular diagnosis of VAP available at your institution? | 34 | ||
| • No | 18 | 52.9 | |
| • Only some very specific techniques and in a timely manner | 12 | 35.3 | |
| • Frequently, for the detection of MRSA, Acinetobacter or other microorganisms | 4 | 11.8 | |
| Indicate LRT samples processed for filamentous fungi: | 34 | ||
| • All samples routinely | 6 | 17.6 | |
| • If requested | 16 | 47.1 | |
| • Certain samples/patients | 12 | 35.3 | |
| Are direct microscopy exams for fungi routinely performed? | 33 | ||
| • No, unless requested | 23 | 69.7 | |
| • Yes, calcofluor-white mount | 5 | 15.2 | |
| • Yes, KOH mount | 3 | 9.1 | |
| • Another method | 2 | 6.1 | |
| Do you perform galactomannan antigen testing? | 34 | ||
| • No | 5 | 14.7 | |
| • Serum galactomannan in sent to a reference laboratory | 12 | 35.3 | |
| • Yes, in serum samples | 8 | 23.5 | |
| • Yes, in serum and BAL samples | 9 | 26.5 | |
| How is the isolation of Candida spp. in LRT samples from patients with suspected VAP interpreted? | 34 | ||
| • Yeasts are inhabitants of the mouth and not a primary cause of pneumonia | 3 | 8.8 | |
| • It is reported qualitatively. No diagnostic interpretation is offered | 7 | 20.6 | |
| • It is reported qualitatively. Caution note not to over-interpret | 11 | 32.4 | |
| • It is reported quantitatively. Value is given to high counts | 13 | 38.2 | |
| How is a diagnosis of herpesviruses (HSV, CMV, etc.) as causative agents of VAP interpreted? | 34 | ||
| • No herpes virus diagnosis is performed due to lack of resources | 16 | 47.1 | |
| • We do have the resources but it is rarely requested | 13 | 38.2 | |
| • Herpes virus diagnosis is frequently performed | 4 | 11.8 | |
| • No diagnosis is performed as its clinical meaning is unclear | 1 | 2.9 | |
| What level of standardization is followed in sample processing and when issuing results? | 34 | ||
| • There are standard operating procedures (STPs) | 31 | 91.2 | |
| • There are STPs though they are not always followed | 2 | 5.9 | |
| • There are no written procedures, but almost always a correct and homogeneous procedure is followed | 1 | 2.9 | |
| Do you periodically prepare an epidemiological report for your ICUs? | 34 | ||
| • Hardly ever | 3 | 8.8 | |
| • Only if requested | 6 | 17.6 | |
| • Yes, annually | 15 | 44.1 | |
| • Yes, twice a year | 5 | 14.7 | |
| • Yes, quarterly | 2 | 5.9 | |
| • Yes, every 1–2 months | 3 | 8.8 | |
| To whom is this report sent? | 34 | ||
| • It is not sent | 4 | 11.8 | |
| • To the hospital committee on infection control | 6 | 17.6 | |
| • To ICUs | 8 | 23.5 | |
| • To both the above mentioned | 16 | 47.1 | |
| Do you consider that present national or international recommendations for the microbiological diagnosis of VAP are satisfactory? | 34 | ||
| • No | 16 | 47.1 | |
| • Yes | 14 | 41.2 | |
| • Maybe/Do not know | 4 | 11.8 | |
| Do you think a multidisciplinary work group should be created under the auspices of the corresponding national scientific societies to pursue a consensus approach to the etiological and microbiological diagnosis of VAP? | 34 | ||
| • Yes | 34 | 100 | |
| If so, would you be willing to collaborate in future studies and work groups with this goal? | 34 | ||
| • Yes | 29 | 85.3 | |
| • No, I have no time | 1 | 2.9 | |
| • I have not decided yet | 4 | 11.6 |
Intensive care unit data. Quantitative variables.
| Item | Valid number | Median | P25 | P75 | Sum |
|---|---|---|---|---|---|
| Number of beds | 37 | 14 | 11.5 | 22.5 | 673 |
| Admissions in the previous year | 32 | 819.5 | 518.25 | 1139.75 | 32,020 |
| Stays in the previous year | 30 | 4606 | 2865.75 | 7114 | 173,820 |
| Patients on mechanical ventilation (min. 24h) in the previous year | 27 | 196 | 116 | 444 | 8305 |
| If information for the whole year is missing but you have data for at least one quarter, please indicate this | 8 | 53.5 | 29.5 | 108.75 | 701 |
| Total number of days of mechanical ventilation in the previous year | 23 | 1800 | 720 | 4192 | 61,268 |
| If information for the whole year is missing but you have data for at least one quarter, please indicate this | 9 | 528 | 404.5 | 2007 | 9826 |
| VAP episodes in the previous year | 23 | 25 | 10 | 48 | 634 |
| LRT samples sent to the laboratory within one hour of collection (%) | 30 | 77.5 | 40 | 90 | 1970 |
| LRT samples sent to the laboratory 1–6h after collection (%) | 30 | 10 | 3.75 | 33.8 | 685 |
| LRT samples sent to the laboratory 7–24h after collection (%) | 30 | 0 | 0 | 6 | 153 |
| LRT samples sent to the laboratory >24h after collection (%) | 30 | 0 | 0 | 0 | 67 |
| Turnaround time (TAT) for preliminary susceptibility testing (days) | 34 | 2.5 | 2 | 3 | 92 |
| Increase in TAT for preliminary susceptibility testing because of a weekend (days) | 36 | 2 | 1 | 2 | 54 |
| TAT for a positive culture (days) | 36 | 4 | 3 | 5 | 141 |
| Increase in TAT for a positive culture because of a weekend (days) | 34 | 2 | 1 | 2 | 57 |
| Reasons why microbiological diagnosis of VAP is impaired at your institution (0=no impact, 10=very high impact) | |||||
| • Lack of nurses | 34 | 2 | 0 | 7 | 118 |
| • Lack of staff | 34 | 2 | 0 | 6 | 103 |
| • Lack of coordination between microbiology and ICU depts. | 34 | 3 | 1.5 | 5.3 | 122 |
| • Lack of coordination within the ICU | 34 | 2.5 | 1 | 5.3 | 107 |
| • Lack of resources | 34 | 3 | 0.8 | 7 | 128 |
| • Lack of microbiology services 24/7 | 33 | 6 | 2 | 8 | 174 |
| • Lack of motivation | 34 | 4 | 2 | 5 | 145 |
| • Lack of standardized diagnostic procedures | 34 | 4 | 2 | 8 | 152 |
| • Lack of correlation between microbiological findings and patient clinical status | 34 | 3 | 1.8 | 6.3 | 128 |
| • TAT too long | 34 | 5 | 2.8 | 7 | 166 |
| • Difficulty in microbiological result interpretation | 34 | 3 | 1 | 6 | 120 |
Intensive care unit data. Qualitative variables.
| Item | Valid number | n | % |
|---|---|---|---|
| Nature of your ICU: | 37 | ||
| • Medical | 5 | 13.5 | |
| • Surgical | 4 | 10.8 | |
| • Medical/surgical | 28 | 75.7 | |
| Which of the following options best describes how VAP is managed at your ICU? | 37 | ||
| • The intensivist manages VAP guided exclusively by microbiological results | 5 | 13.5 | |
| • The intensivist manages VAP usually guided by microbiological results | 32 | 86.5 | |
| Role of the infectious diseases consultant in your ICU: | 36 | ||
| • Treats all VAP episodes | 1 | 2.8 | |
| • Helps treat VAP episodes | 2 | 5.6 | |
| • Intensivists treat VAP episodes aided by the ID consultant | 4 | 11.1 | |
| • The ID consultant is occasionally asked | 10 | 27.8 | |
| • VAP episodes are treated by intensivists alone | 19 | 52.8 | |
| Is there an intensivist specialized in infectious diseases in your unit? | 37 | ||
| • No | 15 | 40.5 | |
| • Yes. Occasionally asked to manage VAP episodes | 11 | 29.7 | |
| • Yes. Manages complicated VAP episodes | 8 | 21.6 | |
| • Yes. Manages all infections in the ICU | 3 | 8.1 | |
| What level of standardization is followed in sample collection and results interpretation in your ICU? | 36 | ||
| • There are standard operating procedures (STPs) | 10 | 27.8 | |
| • There are STPs though they are not always followed | 7 | 19.4 | |
| • There are no written procedures, but almost always a correct and homogeneous procedure is followed | 18 | 50 | |
| • Each physician follows his own procedure | 1 | 2.8 | |
| If you follow a STP, what is it based on? | 30 | ||
| • American Thoracic Society guidelines | 10 | 33.3 | |
| • National recommendations | 5 | 16.7 | |
| • We have our own procedure | 7 | 23.3 | |
| • There is no STP | 8 | 26.7 | |
| Indicate the LRT sample used to diagnose a non-complicated VAP episode: | 37 | ||
| • Blind tracheobronchial aspirate | 3 | 89.2 | |
| • Fiberoptic-guided tracheobronchial aspirate | 1 | 2.7 | |
| • Fiberoptic-guided bronchoalveolar lavage | 1 | 2.7 | |
| • Fiberoptic-guided protected brush specimen | 1 | 2.7 | |
| • Blind PBS | 1 | 2.7 | |
| Indicate the LRT sample used to diagnose a complicated VAP episode: | |||
| • Blind tracheobronchial aspirate | 9 | 24.3 | |
| • Fiberoptic-guided tracheobronchial aspirate | 8 | 21.6 | |
| • Fiberoptic-guided bronchoalveolar lavage | 12 | 32.4 | |
| • Mini bronchoalveolar lavage (blind) | 1 | 2.7 | |
| • Fiberoptic-guided protected brush specimen | 6 | 16.2 | |
| • Blind PBS | 1 | 2.7 | |
| Are surveillance cultures performed to predict the microbial etiology of VAP? | 37 | ||
| • No | 18 | 48.6 | |
| • Yes, once or twice per week | 14 | 37.8 | |
| • Yes, though rarely | 5 | 13.5 | |
| Who collects non-invasive LRT samples? | 37 | ||
| • Nurses | 37 | 100 | |
| When are invasive LRT samples collected? | 37 | ||
| • Only in selected cases when VAP diagnosis is difficult | 16 | 43.2 | |
| • Whenever non-invasive samples have not provided an etiology | 13 | 35.1 | |
| • Often, almost in all patients with suspected VAP | 8 | 21.6 | |
| Is sample delivery to the microbiology laboratory regular? | 35 | ||
| • No. Delivery is delayed in the evening/night | 8 | 22.9 | |
| • Yes | 27 | 77.1 | |
| Indicate the reason why delivery could be delayed: | 28 | ||
| • Reception hours for samples at the microbiology laboratory | 11 | 39.3 | |
| • Lack of personnel in the ICU | 4 | 14.3 | |
| • Other reason | 13 | 46.4 | |
| If sample delivery is delayed, how are samples stored? | 30 | ||
| • At room temperature | 10 | 33.3 | |
| • At 2–4°C | 13 | 43.3 | |
| • Incubated at 35°C | 2 | 6.7 | |
| • Unknown | 5 | 16.7 | |
| Is there a rapid diagnosis for VAP available at your hospital (Gram stain, etc.)? | 37 | ||
| • Yes, if previously requested by phone | 13 | 35.1 | |
| • Yes, if indicated in the laboratory printed/online request form | 21 | 56.8 | |
| • There is no such possibility | 2 | 5.4 | |
| • Unknown | 1 | 2.7 | |
| How do you receive rapid microbiological tests results? | 36 | ||
| • Phone call from the microbiology service | 19 | 52.8 | |
| • We call the microbiology service | 10 | 27.8 | |
| • Through internet | 3 | 8.3 | |
| • We receive a printed report | 3 | 8.3 | |
| • There are no after-hour microbiology reports | 1 | 2.8 | |
| How do you interpret the isolation of Candida spp. in LRT samples from patients with suspected VAP? | 36 | ||
| • No value whatsoever | 4 | 11.1 | |
| • It indicates colonization | 18 | 50 | |
| • It is a causative agent of pneumonia | 1 | 2.8 | |
| • It is a causative agent of pneumonia in special situations (e.g., in severely ill patients, invasive samples, etc.) | 13 | 36.1 | |
| How do you interpret the isolation of Aspergillus spp. in LRT samples from patients with suspected VAP? | 36 | ||
| • It is a causative agent of pneumonia only in immunocompromised patients | 20 | 55.6 | |
| • Its isolation from a LRT sample may indicate invasive Aspergillosis in any patient | 16 | 44.4 | |
| What is your stand on herpes viruses (HSV, CMV, etc.) as agents of VAP in immunocompetent patients?: | 35 | ||
| • I never request herpes virus diagnosis because their pathogenicity is uncertain | 11 | 31.4 | |
| • I believe in their pathogenicity but diagnosis is not available | 4 | 11.4 | |
| • If occasionally requested, their diagnosis is available but their role in VAP is uncertain | 11 | 31.4 | |
| • Their diagnosis is available and their isolation influences treatment | 9 | 25.7 | |
| Who collects non-invasive LRT samples? | 37 | ||
| • Nurses | 37 | 100 | |
| When are invasive LRT samples collected? | 37 | ||
| • Only in selected cases when VAP diagnosis is difficult | 16 | 43.2 | |
| • Whenever non-invasive samples have not provided an etiology | 13 | 35.1 | |
| • Often, almost in all patients with suspected VAP | 8 | 21.6 | |
| Is sample delivery to the microbiology laboratory regular? | 35 | ||
| • No. delivery is delayed in the evening/night | 8 | 22.9 | |
| • Yes | 27 | 77.1 | |
| Indicate the reason why delivery could be delayed: | 28 | ||
| • Reception hours for samples at the microbiology laboratory | 11 | 39.3 | |
| • Lack of personnel in the ICU | 4 | 14.3 | |
| • Other reason | 13 | 46.4 | |
| If sample delivery is delayed, how are samples stored? | 30 | ||
| • At room temperature | 10 | 33.3 | |
| • At 2–4°C | 13 | 43.3 | |
| • Incubated at 35°C | 2 | 6.7 | |
| • Unknown | 5 | 16.7 | |
| Is there a rapid diagnosis for VAP available at your hospital (Gram stain, etc.)? | 37 | ||
| • Yes, if previously requested by phone | 13 | 35.1 | |
| • Yes, if indicated in the laboratory printed/online request form | 21 | 56.8 | |
| • There is no such possibility | 2 | 5.4 | |
| • Unknown | 1 | 2.7 | |
| How do you receive rapid microbiological tests results? | 36 | ||
| • Phone call from the microbiology service | 19 | 52.8 | |
| • We call the microbiology service | 10 | 27.8 | |
| • Through internet | 3 | 8.3 | |
| • We receive a printed report | 3 | 8.3 | |
| • There are no after-hour microbiology reports | 1 | 2.8 | |
| How do you interpret the isolation of Candida spp. in LRT samples from patients with suspected VAP? | 36 | ||
| • No value whatsoever | 4 | 11.1 | |
| • It indicates colonization | 18 | 50 | |
| • It is a causative agent of pneumonia | 1 | 2.8 | |
| • It is a causative agent of pneumonia in special situations (e.g., in severely ill patients, invasive samples, etc.) | 13 | 36.1 | |
| How do you interpret the isolation of Aspergillus spp. in LRT samples from patients with suspected VAP? | 36 | ||
| • It is a causative agent of pneumonia only in immunocompromised patients | 20 | 55.6 | |
| • Its isolation from a LRT sample may indicate invasive aspergillosis in any patient | 16 | 44.4 | |
| What is your stand on herpes viruses (HSV, CMV, etc.) as agents of VAP in immunocompetent patients?: | 35 | ||
| • I never request herpes virus diagnosis because their pathogenicity is uncertain | 11 | 31.4 | |
| • I believe in their pathogenicity but diagnosis is not available | 4 | 11.4 | |
| • If occasionally requested, their diagnosis is available but their role in VAP is uncertain | 11 | 31.4 | |
| • Their diagnosis is available and their isolation influences treatment | 9 | 25.7 | |
| Are quantitative (cfu/mL) cultures performed in LRT samples? | 35 | ||
| • Always | 22 | 62.9 | |
| • If requested | 3 | 8.6 | |
| • If the microbiology service deems it appropriate | 3 | 8.6 | |
| • No | 7 | 20 | |
| What is the threshold for VAP diagnosis in tracheal aspirates? | 34 | ||
| • >106cfu/mL | 9 | 26.5 | |
| • >105cfu/mL | 21 | 61.8 | |
| • >103cfu/mL | 1 | 2.9 | |
| • There is no threshold | 2 | 5.9 | |
| • This type of sample is infrequently collected | 1 | 2.9 | |
| What is the threshold for VAP diagnosis in bronchial aspirates? | 32 | ||
| • >106cfu/mL | 5 | 15.6 | |
| • >105cfu/mL | 16 | 50 | |
| • >104cfu/mL | 6 | 18.8 | |
| • >103cfu/mL | 2 | 6.3 | |
| • There is no threshold | 1 | 3.1 | |
| • This type of sample is infrequently collected | 2 | 6.3 | |
| What is the threshold for VAP diagnosis in bronchioalveolar lavage? | 32 | ||
| • >106cfu/mL | 1 | 3.1 | |
| • >105cfu/mL | 3 | 9.4 | |
| • >104cfu/mL | 21 | 65.6 | |
| • >103cfu/mL | 5 | 15.6 | |
| • There is no threshold | 1 | 3.1 | |
| • This type of sample is infrequently collected | 1 | 3.1 | |
| What is the threshold for VAP diagnosis in protected brush specimens? | 31 | ||
| • >106cfu/mL | 1 | 3.2 | |
| • >105cfu/mL | 1 | 3.2 | |
| • >104cfu/mL | 2 | 6.5 | |
| • >103cfu/mL | 19 | 61.3 | |
| • >102cfu/mL | 1 | 3.2 | |
| • There is no threshold | 1 | 3.2 | |
| • This type of sample is infrequently collected | 6 | 19.4 | |
| What is the value of quantification when interpreting culture results? | 35 | ||
| • It may help interpret results | 5 | 14.3 | |
| • It is important to interpret results | 18 | 51.4 | |
| • It is of paramount importance. Microorganisms appearing in high numbers are the causative agents of VAP | 12 | 34.3 | |
| Dou you periodically receive an epidemiological report in your ICU? | 36 | ||
| • No | 3 | 8.3 | |
| • We have our own database | 7 | 19.4 | |
| • If we request it from the microbiology service | 4 | 11.1 | |
| • Yes, from the Preventive Medicine service or the infection control committee | 10 | 27.8 | |
| • Yes, from the microbiology service | 12 | 33.3 | |
| What is the usefulness of microbiological information in the diagnosis and management of VAP? | 37 | ||
| • Very useful | 33 | 89.2 | |
| • Useful | 4 | 10.8 | |
| Do you consider that present national or international recommendations for the microbiological diagnosis of VAP are satisfactory? | 36 | ||
| • No | 13 | 36.1 | |
| • Yes | 21 | 58.3 | |
| • Maybe/Do not know | 2 | 5.6 | |
| Do you think a multidisciplinary work group should be created under the auspices of the corresponding national scientific societies to pursue a consensus approach to the etiological and microbiological diagnosis of VAP? | 36 | ||
| • Yes | 36 | 100 | |
| If so, would you be willing to collaborate in future studies and work groups with this goal? | 36 | ||
| • Yes | 33 | 89.2 | |
| • I have not decided yet | 3 | 8.1 |
One questionnaire was addressed to microbiologists (Tables 1 and 2) and another to intensivists (Tables 3–4). Some questions were common to both questionnaires to separately assess the opinions of both on the same issue.
Microbiologists were asked about the size, structure and activity of their hospital and department, including personnel. Data were compiled regarding respiratory samples obtained from critically ill patients such as number, type and processing of these samples. Information was also obtained on turnaround times for rapid and standard diagnostic tests, guidelines for the interpretation of microbiological results, preparation of epidemiological records, and present recommendations for VAP diagnosis.
Intensivists answered questions about the characteristics of their intensive care units (ICUs) and their protocols for VAP diagnosis (sample collection, storage and delivery). They were also asked about turnaround times for rapid and standard diagnostic tests, guidelines for the interpretation of microbiological results, reception of epidemiological records from the Microbiology Department, and present recommendations for VAP diagnosis.
Finally both microbiologists and interventionists were asked on their opinion about the need for a consensus approach to the microbiological diagnosis of VAP.
A web site (http://denevem.com) was designed with the questionnaires available for electronic completion. Access to the web site was restricted to participating members by means of a personal username and password.
Ethical issuesNo individual patient data were collected. The study protocol was approved by our institution's review board. The need for informed consent was waived.
StatisticsCategorical variables are provided with their frequency distributions. Continuous variables are summarized as medians and inter-quartile ranges (IQR). Data were recorded in an SPSS database (SPSS ver. 18.0, SPSS, Chicago, Illinois, USA).
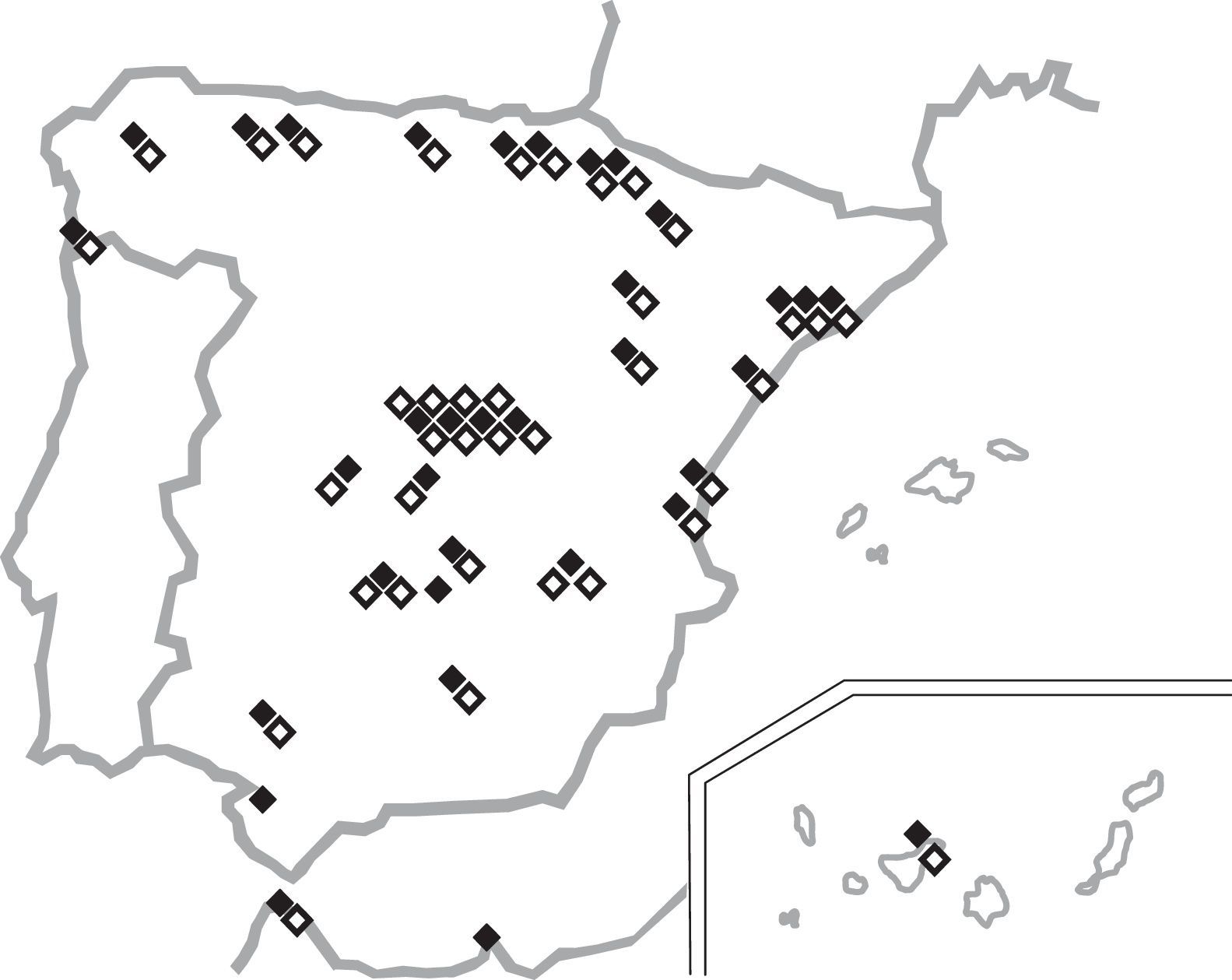
ResultsThirty-four microbiology (42.5%) and thirty-seven ICU (46.3%) departments belonging to thirty-five Spanish hospitals agreed to participate in the study. Of these, 94% were public or mixed (public-private) institutions (only 2 were private centers). The population covered by the participating hospitals was approximately 10million inhabitants (range 50,819–750,000 per cent). The total number of available beds was 21,524 (range 110–1575 per center). Hospital admissions in the previous year were 857,631 and these generated 5,432,443 days of stay. Participating ICUs had 673 beds in total (range 6–48 per ICU); and 32,020 admissions and 173,820 days of stay over the whole year. Questionnaires were answered by attending physicians in 94% and 89% of the Microbiology and ICU departments, respectively, thus reflecting present working practices in those units. The locations and characteristics of the participating departments are shown in Tables 1 and 2 and Fig. 1. The median VAP incidence density was 8.7 episodes per 1000 ventilator-days (IQR 6.5–13.9).
Relevant information from the microbiology (Tables 1 and 2) and ICU (Tables 3 and 4) departments is indicated below.
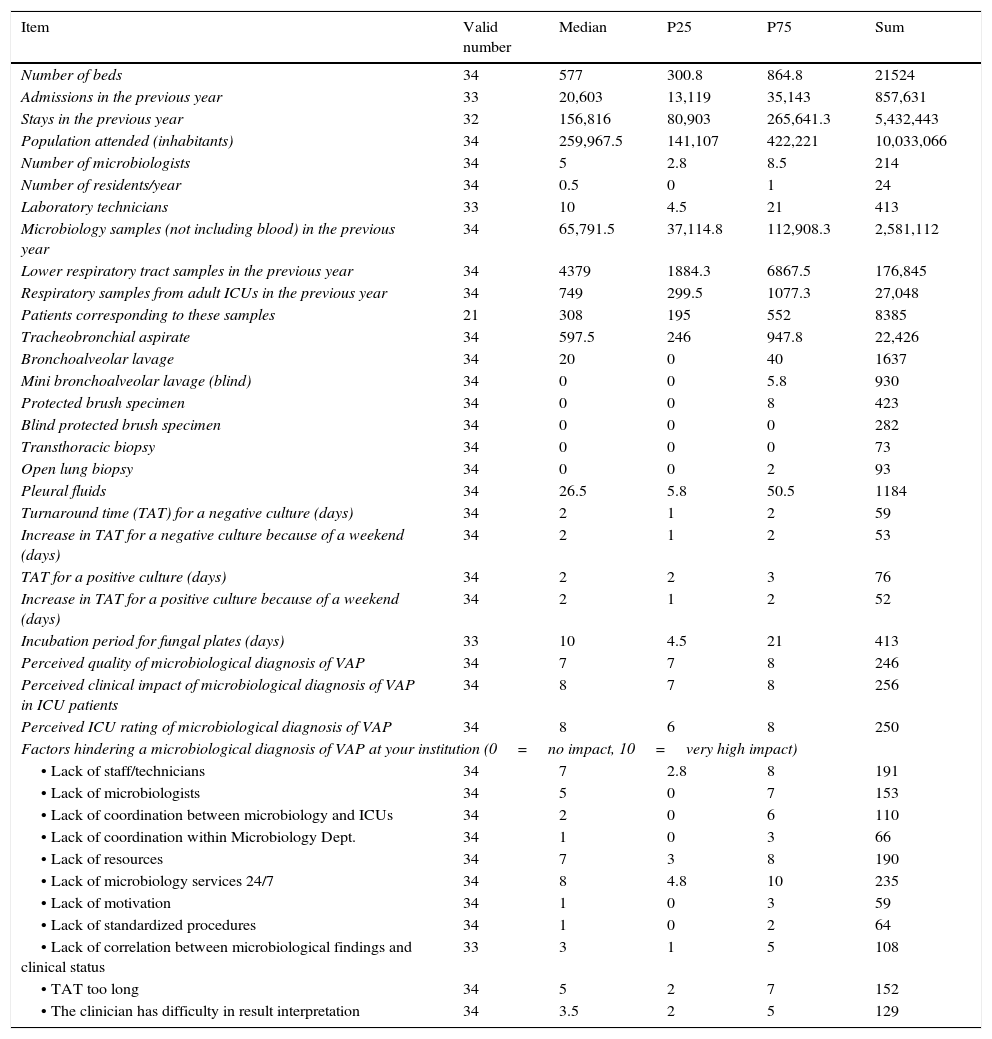
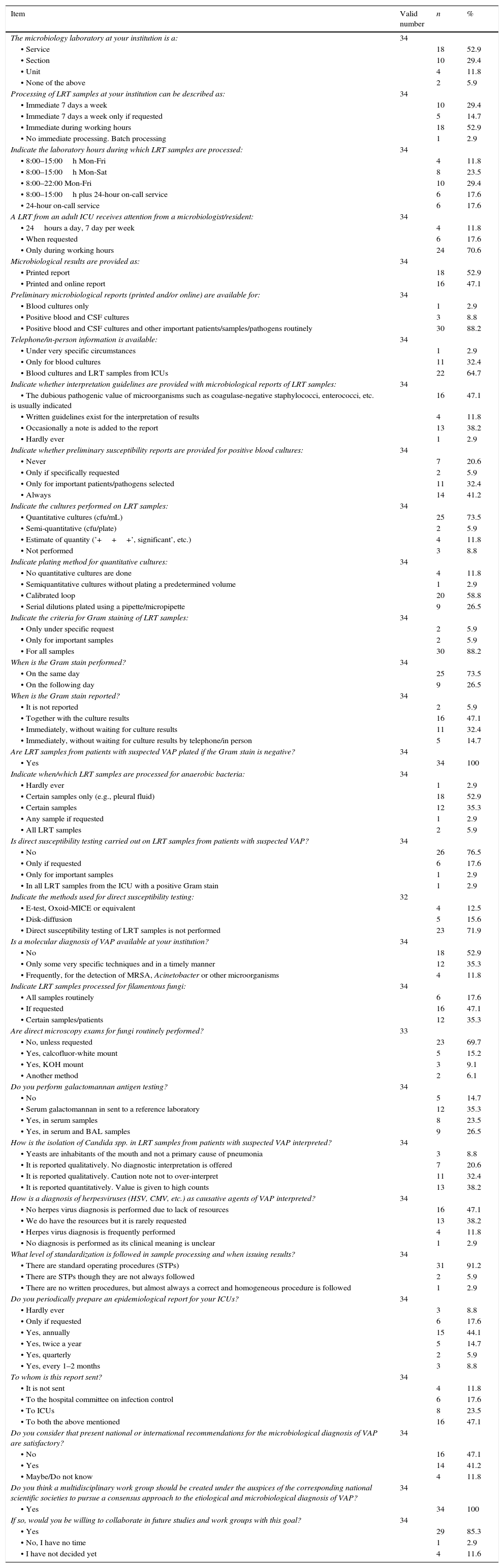
Participating microbiology departments received 27,048 lower respiratory tract (LRT) samples during the previous year from adult ICUs, representing 15.3% of all LRT samples received, 844.7 LRT samples per 1000 ICU admissions and 1556.1 LRT samples per 10,000 days of ICU stay. These samples were immediately processed only in 29.4% of the hospitals, all of which had microbiology services available on a 24/7 basis. Most LRT samples were tracheal aspirates (82.9%). According to the microbiologists questioned, Gram staining was available in 88.2% of the laboratories but only 47.1% reported results immediately; in 5.9% of cases, Gram staining results were never reported. Molecular diagnostic techniques were routinely directly applied to LRT samples in 4 institutions (11.8%); if specifically requested, this could be done in a timely manner in a further 35.3% of centers. Only one center routinely undertook direct antimicrobial susceptibility testing on LRT samples from ICU patients with suspicion of VAP when microorganisms were seen in the Gram stain. This practice was nevertheless feasible in a further 17.6% of centers if requested by the intensivist. LRT samples were not usually tested for filamentous fungi, but when this was done, direct microscopy exams were not performed on a regular basis. The detection of galactomannan antigen in bronchoalveolar lavage (BAL) samples was available only in 26.5% of the microbiology departments. The diagnosis of herpesvirus in LRT samples was available in 50% of participating hospitals.
Quantitation of LRT samples was performed in 73.5% of the cases, mostly using a calibrated loop or pipette to plate serial dilutions (85.3%). Candida spp. were reported quantitatively and high counts (those above the diagnostic threshold) were considered significant in 38.2% of the departments.
Mean turnaround times (TAT) were 1.7 (SD 0.7) days for a microbiological report of a negative result and 2.2 (SD 0.6) for a positive result. These figures were increased by a mean 1.6 (SD 0.7) days if there was a weekend involved. Microbiological results were issued exclusively as a printed report in 52.9% of the departments. Telephone/in-person information about culture results of LRT samples from ICUs was offered by 64.7% of the hospitals. In 73.5% of the institutions, an epidemiological report for ICUs was provided on a regular basis.
The perceived quality of the microbiologists’ own microbiological diagnosis of VAP was rated on a scale from 0 (very poor) to 10 (excellent) as a median of 7 points (IQR 7–8). The perceived clinical impact of this diagnosis was awarded a median score of 8 (IQR 7–8). When asked about the opinion that their ICU colleagues had about the microbiological diagnosis of VAP, 8 points (IQR 6–8) were given. Microbiologists considered that the main factors hindering a microbiological diagnosis of VAP were a lack of staff/technicians (median 7 points, IQR 2.8–8), lack of resources (median 7 points, IQR 3–8) and lack of a microbiology service 24/7 (median 8 points, IQR 4.8–10). Almost half (47.1%) of the microbiologists considered that present recommendations for the microbiological diagnosis of VAP are insufficient and mentioned they would be willing to form part of a work group to establish a revised consensus approach to the etiological and microbiological diagnosis of VAP.
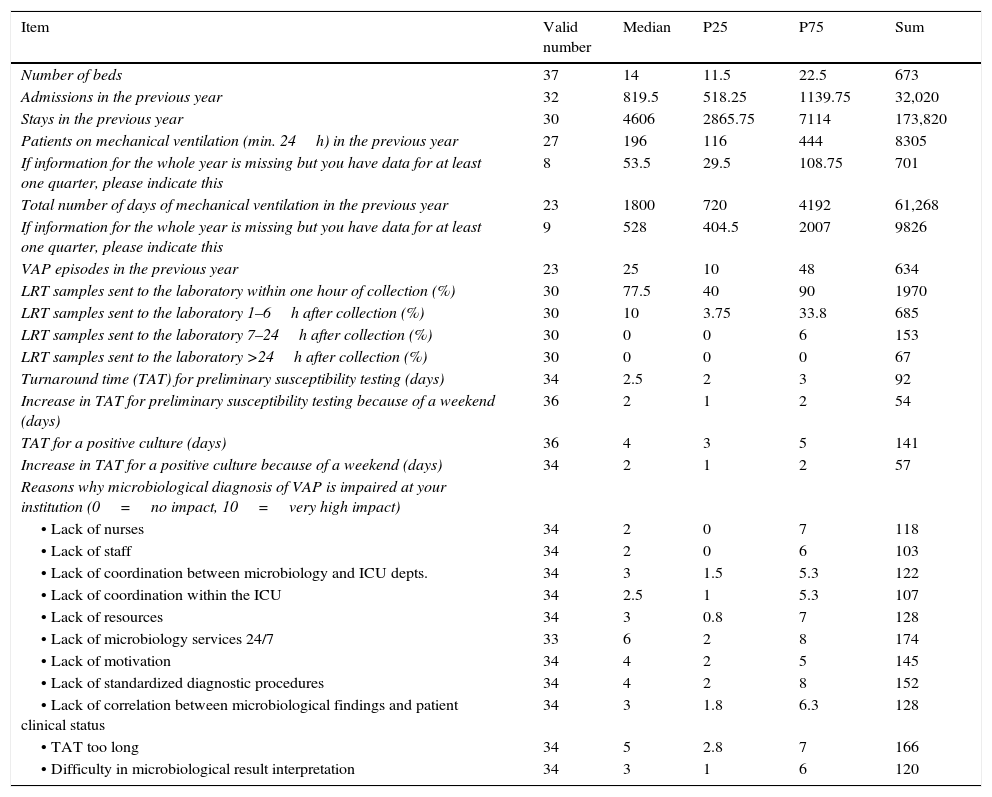
The data from the questionnaire administered to ICU departments (Tables 3 and 4) revealed that tracheal aspirates were the first samples most frequently collected for VAP diagnosis (89.2%). Bronchoscopy was only used in patients when a non-invasive sample had not provided an etiologic diagnosis, or with complicated or non-responding VAP.
Only 38% of the ICU departments admitted they performed surveillance LRT cultures once or twice per week. When asked about sample delivery to the microbiology department, intensivists reported this was done within one hour of collection in a median of 77.5% (IQR 40–90) of the ICUs. Sample submission to the laboratory was delayed in the evenings/nights in 22.9% of the departments, mainly (39.3%) due to limited reception hours in the Microbiology Department. In these cases, samples were improperly stored (room temperature, incubated at 35°C) in 40% of the ICUs.
Intensivists used the following bacterial thresholds for a diagnosis of VAP: >105cfu/mL for tracheal and bronchial aspirates (61.8% and 50%, respectively), >104cfu/mL for BAL (65.6%) and >103cfu/mL for a protected brush specimen (61.3%). As many as 85.7% of intensivists considered that quantitative culture results were very useful and 89.2% highly valued microbiological information for a diagnosis of VAP.
According to intensivists, the median TAT for preliminary susceptibility testing results was 2.5 days (IQR 2–3). The median time for a positive culture result was 4 days (IQR 3–5) and this increased to a median of 2 days (IQR 1–2) if there was a weekend involved.
Intensivists agreed with the microbiologists questioned that Candida spp. are responsible for pneumonia only in very few patients and when recovered from truly invasive samples (36.1%). The isolation of Aspergillus spp. was only considered significant in immunosuppressed patients (55.6% of cases). Most intensivists (62.8%) consider that the pathogenicity of herpesvirus in VAP in immunocompetent patients is uncertain, although 25.7% prescribed specific treatment when herpes virus was diagnosed.
In 52.8% of the hospitals, VAP episodes were routinely managed by intensivists alone without infectious disease (ID) consultation.
The questionnaires completed by intensivists revealed that no written procedures on sample collection and microbiological result interpretation were available in 50% of the departments. When written procedures were available, these were based on different guidelines (ATS 33.3%, national 16.7% and locally adapted 23.3%).
Intensivists mentioned that the main factors hindering a microbiological diagnosis of VAP were the lack of 24/7 microbiology services (median 6 points, IQR 2–8) and the long turnaround times of microbiological reports (median 5 points, IQR 2.8–7). One third (36.1%) of the intensivists reported that present recommendations for a microbiological diagnosis of VAP are imprecise and like the microbiologists stated they would be more than willing to help establish a set of detailed consensus guidelines for the etiological and microbiological diagnosis of VAP.
DiscussionThe results of our survey reveal different approaches to the diagnosis of VAP in ICUs across Spain and serve to identify areas for improvement. As reported in the literature, diagnostic techniques for VAP vary widely10 and guidelines for its diagnosis clarifying the role of the Microbiology Department are urgently required.
Unfortunately, we still lack a gold standard for VAP diagnosis. In this age of evidence-based medicine, guidelines play an important role in the diagnosis and management of life threatening diseases such as VAP. Guidelines for VAP have two primary goals: to reduce its overall incidence through preventive strategies, and to improve its morbidity and mortality by recommending appropriate management and treatment.11
When making a rapid microbiological diagnosis of VAP, only a third of ICUs had access to a laboratory with a 24/7 schedule. A Gram stain result was immediately obtained only in half of the hospitals. This contradicts present guidelines, which warn that LRT samples from patients with a suspicion of VAP should be analyzed within 2–4h of collection8 and that Gram stain findings should be immediately reported to aid in the diagnosis and management of VAP.12 Most clinicians argue that the average turnaround time for ID screening to have an impact on medical practice is about 2h for inpatients.13 The availability of a microbiology service on a 24/7 basis is therefore an essential requirement.
The most frequent LRT sample identified in the survey was a blind tracheal aspirate, consistent with most published reports.14–16 Invasive samples were reserved for patients in whom VAP diagnosis was difficult or whenever non-invasive samples had failed to provide an etiology. A microbiological diagnostic standard for VAP is still missing, reflecting the particularly poor quality of evidence in this area. No differences in mortality were detected when invasive or non-invasive samples were used to diagnose VAP.9,17 However, the invasive approach almost consistently prompts changes in the antibiotic regimen resulting in de-escalation and shorter antibiotic courses.18–20 Still, until better evidence emerges, the use of invasive bronchoscopic techniques is not recommended for the routine diagnosis of VAP.21
In the majority of hospitals, LRT samples were quantitatively plated. According to the thresholds put into practice, 18.8% of intensivists considered 104–105cfu/mL positive for a tracheobronchial sample and 15.6% considered 103–104cfu/mL positive for a protected brush specimen (PBS), likely overdiagnosing this disease. In a recent review of published data regarding the use of quantitative versus qualitative LRT cultures in patients with VAP published by the Cochrane Database of Systematic Reviews, no evidence was found that quantitative cultures resulted in reduced mortality, reduced ICU stay and time on mechanical ventilation, or in higher rates of antibiotic change.22 Similar results were obtained when invasive strategies were compared with non-invasive strategies.
The diagnostic approach to VAP seems up to the discretion of the clinician responsible for the patient. Factors to consider include local experience, expertise, availability, and cost. What does seem clear is that a timely microbiological diagnosis helps establish adequate antibiotic therapy, de-escalation,23 and reduces costs, as therapy may be tailored to less expensive antibiotics once culture results are available.1
The value of surveillance cultures has also been called into question. This practice is performed in half the hospitals of our survey. Existing evidence of the value of surveillance cultures is far from conclusive. The few studies addressing this question, besides including small patient numbers, have involved varying methods and different frequencies of sampling.24,25 In the studies by Delclaux and Boots, the sensitivity, specificity, and positive and negative predictive values of surveillance cultures ranged from 67 to 84%, 28.5 to 50%, 31 to 89% and 60 to 93%, respectively.26,27 In a study by Bouza and co-workers in patients undergoing heart surgery,28 the sensitivity of surveillance cultures was unacceptably low to justify their systematic use. It remains to be seen whether surveillance cultures allow for better empirical antibiotic therapy than the use of current treatment guidelines for this entity. As more reports solidify their clinical usefulness, cost is still probably the most important factor preventing the general use of systematic surveillance cultures.
With regards to the pathogenicity of specific microorganisms, for instance in the case of Candida spp., the American Society of Microbiology recommends their isolation be reported only if there is a heavy amount of pure growth from BAL or PBS cultures in certain immunocompromised patients (e.g., leukemia patients, solid organ transplant recipients or neonates).29 Despite this, a third of the microbiologists participating in our survey routinely informed of its isolation in a quantitative manner, and intensivists also took this information into account.
There are studies in which the isolation of Aspergillus spp. from LRT samples in ICU patients is representative of pulmonary aspergillosis in only half of the patients, as in our study.30–32 The diagnosis of pulmonary aspergillosis is not easy in this population, and there is no universally accepted definition, apart from the histologic diagnosis in lung tissue. In a recent multicenter Spanish study that prospectively collected data on ICU-acquired infections related to invasive devices, ICU patients were considered to have a pulmonary aspergillosis if Aspergillus spp. was isolated from two or more respiratory samples, patients presented with risk factors for this infection (mainly immunosuppression), and they did not respond to treatment with broad-spectrum antibiotics.33 In this study, it was found that up to 25% of patients with isolation of Aspergillus spp. from TRI samples received no antifungal treatment (or this had not been recorded), probably reflecting colonization/contamination and not a true infection. It is evident that for the diagnosis of this entity is not enough to have positive LRT cultures, given the ubiquity of this microorganism and its ability to colonize clinical samples and to contaminate culture plates.
The role of respiratory viruses in VAP remains uncertain. Some published data provide evidence that Herpesviridae could be associated with a more severe disease and adverse clinical outcome,34,35 though their specific role in the development of VAP remains to be defined. These qualms are reflected in our survey, in which few centers rarely attempted their diagnosis in these patients.
Our survey revealed that microbiology results were only sent as a printed report in the majority of hospitals, contributing to long turnaround times. Reporting selected results by telephone together with reasonably fast results is considered important for a quality service.36 In the centers included in our survey, turnaround times were significantly lengthened when microbiology services were interrupted by a weekend and could be extended to 6 days.
We should highlight that half the participating ICUs had no written protocols for the diagnosis and management of patients with VAP. Written procedures have shown their usefulness in preventing and therefore decreasing rates of VAP,37 catheter-related bloodstream infection38 or surgical site infection.39 Such standard procedures also serve to unify practices in settings such as VAP patient management.40
The microbiologists questioned here considered that the quality of the microbiological diagnosis of VAP at their centers was good having a high clinical impact, and believed that intensivists were satisfied with this quality. They considered that major hurdles for an adequate and timely diagnosis were a lack of personnel and resources and the absence of a microbiology department open 24/7. Microbiologists also considered that turnaround times for LRT samples were too long. Intensivists concurred with these views. Both stated that there is definitely room for improvement and that they would be willing to collaborate in an initiative to help improve the microbiological diagnosis of VAP. The role of microbiologists in the diagnosis and management of VAP is yet to be defined.
A robust diagnosis of VAP is required for patient management, for epidemiology surveillance programs, to measure the effectiveness of preventive measures and for clinical trials. VAP diagnosis has effectively been identified as a key area susceptible to substantial improvement.12
Guidelines play a significant role in improving the diagnosis and management of hospital-acquired infections such as VAP. Guideline implementation is difficult and requires multiple efforts. In a survey investigating why ICU physicians do not follow evidence-based guidelines for VAP, the reasons given were mainly disagreement with the interpretation of clinical trials, lack of resources and costs. The overall non adherence rate was 37%.41
This article is a plea for the more effective implementation of more-to-the-point guidelines that will ultimately benefit patients with VAP.
Conflict of interestThe authors declare no conflict of interest.
The authors thank Cristina Fernández for statistical advice and Ana Burton for editorial assistance.
A. Aguinaga Pérez, J. Leiva León, R. Calderón Pelayo, F. Carrascosa Moreno (Clínica Universitaria de Navarra); J. J. García Irure, C. Ezpeleta Baquedano, J. Insausti, J. Guergué, B. Suberviola Cañas, F. Ortiz Melón (Complejo Hospitalario de Navarra); M. A. Pallarés González, M. García Campello, S. Freita Ramos, C. Galbán Rodríguez (Complejo Hospitalario de Pontevedra); S. García de la Cruz, A. Campos Bueno, M. J. Fernández Calavia, M. Gobernado (Complejo Hospitalario de Soria, Hospital Santa Bárbara); N. Batista Díaz, A. Pacual Hernández, A. Arenzana Seisdedos, T. Guzmán Valencia (Complejo Hospitalario Regional Virgen Macarena); P. Robles Domínguez, M. D. Crespo Sánchez, F. García López, A. Martínez García, J. M. Jiménez Vizuete, R. Peyro García (Complejo Hospitalario Universitario de Albacete); J. Pita Carretero, M. P. Alonso García, J. C. Portela Alvarez (Complejo Hospitalario Universitario Xeral-Calde); J. Puig de la Bellacasa, M. T. Jiménez de Anta, A. Torres (Hospital Clinic, Barcelona); R. Tejero García (Hospital Comarcal de Melilla); R. Villa-Real Berruezo, A. Bartolomé Sanz, A. Laguna Frías (Hospital Comarcal San Juan de la Cruz, Úbeda); G. Viejo de la Guerra, L. Otero Guerra, J. M. Quiroga Ruiz, M. Antuña Braña (Hospital de Cabueñes); A. Sánchez-Porto (Hospital de La Línea de la Concepción); M. Espasa Soley, D. Fontanals Aymerich, J. Vallés, A. Artigas (Hospital de Sabadell); M. D. Romero Aguilera, H. Abdel-Hadi Alvarez, A. Ambros Checa, F. J. Redondo Calvo, G. Bernal García, L. Collar Viñuelas (Hospital General de Ciudad Real); M. Huertas Vaquero, R. Carranza González, J. C. Igeño Cano, J. Serrano Castañeda (Hospital General La Mancha Centro); A. Beteta López, M. T. Gil Ruiz, F. Alba García,P. López Onega (Hospital General Nuestra Señora del Prado); A. Betrán Escartín, M. Ferrero Cáncer, J. L. Labarta Monzón, C. Serón Arbeloa (Hospital General San Jorge, Huesca); P. Martín-Rabadán Caballero, J. Hortal, A. González Pinto, I. Frías, L. Fernández Quero, M. Sancho González, J. E. Guerrero Sanz (Hospital General Universitario Gregorio Marañón); B. Iglesias Rodríguez, P. Prendes Peláez, M. Valledor Méndez, A. Rivera Fernández (Hospital San Agustín, Asturias); F. F. Gómez Bertomeu, J. M. Santamaría Puig, E. Díaz (Hospital Universitari Joan XXIII de Tarragona); A. Ferrer Marcelles, A. Andreu Domingo, J. Rello (Hospital Universitari Vall d’Hebrón); A. Arribi Vilela, J. J. Picazo de la Garza, M. Sánchez García (Hospital Universitario Clínico San Carlos); T. Delgado Melián, M. Lecuona Fernández, L. Lorente Ramos, M. L. Mora Quintero (Hospital Universitario de Canarias); J. López Barba, I. Martínez Bagur (Hospital Universitario de Ceuta); J. Colomina Rodríguez, S. Tormo Ferrándiz, J. Gregori Mompó (Hospital Universitario de la Ribera, Alzira); J. J. Camarena Miñana, J. M. Nogueira Coito, D. C. Tormo Calandín (Hospital Universitario Doctor Peset); D. Vicente, E. Pérez-Trallero, E. Cabarcos Gravalos, P. Marco Garde (Hospital Universitario Donostia); R. Ruiz de Luna González, A. Algora Weber (Hospital Universitario Fundación de Alcorcón); J. Zapardiel, I. Gadea, C. Pérez Calvo, A. Gamo de Maeyer, D. López Mendoza (Hospital Universitario Fundación Jiménez Díaz); M. De Pablos Gómez, A. Gutiérrez Altés, F. Gilsanz Rodríguez, B. Galván, M. Jiménez Lendinez (Hospital Universitario La Paz); C. García de la Fuente, L. Martínez Martínez, B. Suberviola Cañas, F. Ortiz Melón (Hospital Universitario Marqués de Valdecilla); M. C. Villendas Usón, M. J. Revillo Pinilla, V. González Sanz (Hospital Universitario Miguel Servet); A. Sánchez-Maroto Lozano (Hospital Virgen de Altagracia, Manzanares); M. V. Martino Castañar, S. Brea Zubigaray, P. López-Reina Torrijos, M. A. Arrese Cosculluela (Hospital Virgen de la Salud); J. A. Jiménez Alfaro, K. Reviejo Jaka (Policlínica Guipuzkoa).