To evaluate the impact of external urine collection devices (UCD) on contamination of urine samples in women with symptoms of urinary tract infection.
MethodsThis review was conducted according to the Systematic Reviews of Diagnostic Test Accuracy guidelines (PROSPERO CRD42021241758). PubMed was searched for paired sample studies and controlled trials. Studies comparing UCDs with non-invasive urine collection procedures were considered.
ResultsOnly two studies were found. Neither of the two studies found any difference regarding contamination between specimens collected with the UCDs compared and non-invasive techniques. In the largest study, including 1264 symptomatic women, 18.8% of those allocated to UCDs failed to collect urine samples successfully.
ConclusionsMore studies involving women with symptoms of urinary tract infection are needed to produce more robust data on the impact of these devices on urine contamination rates.
Evaluar el impacto de los dispositivos externos de recogida de orina (DERO) sobre la contaminación en muestras de orina en mujeres con síntomas de infección urinaria.
MétodosEsta revisión siguió la pauta de revisiones sistemáticas de pruebas diagnósticas (PROSPERO CRD42021241758). Se realizó una búsqueda en PubMed de estudios de muestras pareadas y ensayos controlados. Se consideraron los estudios que compararon los DERO con procedimientos no invasivos de recogida de orina.
ResultadosSolo se hallaron 2 estudios. Ninguno encontró diferencia alguna en la contaminación de las muestras recogidas con DERO y técnicas no invasivas. En el estudio más grande, que incluyó a 1.264 mujeres sintomáticas, el 18,8% de las asignadas a los DERO no pudieron recoger las muestras satisfactoriamente.
ConclusionesSe necesitan más estudios con mujeres con síntomas de infección urinaria para tener datos más consistentes del impacto de estos dispositivos sobre la contaminación de las muestras urinarias.
Acute urinary tract infections (UTI) are very common among women in general practice. Urine culture is considered the gold standard method for the diagnosis of UTI, but improper sample collection can lead to contamination with normal urogenital commensals, which hamper the quantitative urine analysis.1 Contamination rates vary widely across microbiology labs.2 Despite their usage for urine incontinence mainly in men, the evidence about the impact of external urine collection devices (UCD) on the contamination rate of urine samples in women with UTI symptoms is limited. Nonetheless, several studies have been performed, basically in healthy asymptomatic individuals. There is the assumption that these UCDs could be of particular benefit for women with symptomatic UTI, in whom most urine cultures are requested. Other populations could also benefit from their use, such as pregnant women in the screening for asymptomatic bacteriuria in antenatal care, and in individuals in pre-operative care because of positive results they can have a substantial effect on the care pathway.
Several external UCDs are marketed, but Peezy Midstream Urine UCD (Forte Medical, London) and Whiz Midstream UCD (Oxford Devices, Oxford) are the most common medical devices for midstream urine (MSU) collection.3,4 During urine collection, the first void urine is automatically expelled into the toilet. MSU urine is captured in the collection tube, and excess urine is diverted into the toilet once the tube is full. We conducted the current systematic review aimed at evaluating the impact of external USDs on the contamination rates of urine specimens collected in women with UTI symptoms.
MethodsSearch strategyThis systematic review was conducted in accordance with the Systematic Reviews of Diagnostic Test Accuracy guidelines and the protocol was registered in PROSPERO (CRD42021241758).5 We searched PubMed from the inception until 10 July 2021. A new search was performed on 1 March 2023 for recent papers. Reference lists and citations of included studies were backward searched for additional studies. No restrictions were applied in ways of publication language. The search strategy is described in Appendix 1.
Review questions and outcomes of interestThe population, intervention, comparison, and outcome, known as PICO elements, were as follows: P. the population was constituted of any woman aged 18 or older with symptoms of UTI with urine cultures collected; I. the intervention was clinical practice; C. comparison was made of studies comparing UCDs with exclusively non-invasive sample collections, such as midstream clean-catch (MSCC) with soap and/or water, MSU samples, first-void urine (FVU) samples, home-voided samples with instructions, and random voiding samples; O. the main outcomes were the results in contaminated rates.
Eligibility criteriaFor inclusion, studies needed to have a paired design or be a controlled trial comparing collection of urine with UCD with non-invasive collection methods preferably only in women with symptoms of UTI or in a vast majority of women with symptoms of UTI. Studies were excluded if they compared invasive methods for obtaining a urine sample, such as the use of catheters, suprapubic aspirate, cystoscopy, ureteric, ileal conduit, urostomy or nephrostomy urine. Studies investigating patients who were asymptomatic, pregnant, children and/or men were also excluded. Neither was the use of UCDs for conditions other than symptomatic UTIs, such as urine incontinence, considered. Use of absorbing diapers was neither included.
Screening of title and abstracts, and full text was undertaken independently by A.M and C.L. Any disagreement between reviewers was resolved by discussion. If consensus could not be reached, review was undertaken by another other reviewer (A.G.S.). The same procedure was used for extracting the required information from the primary studies.
Risk of biasThe risk of bias of each study included was assessed by two independent authors (A.M. and C.L.) using the Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2).6 Any disagreement was resolved by discussion. Four components were assessed: patient selection, index test, reference standard, flow, and timing. Each study was scored according to whether the assessment criteria are met or not, and then classified as being of “high risk”, “low risk”, or “unclear risk” of bias.
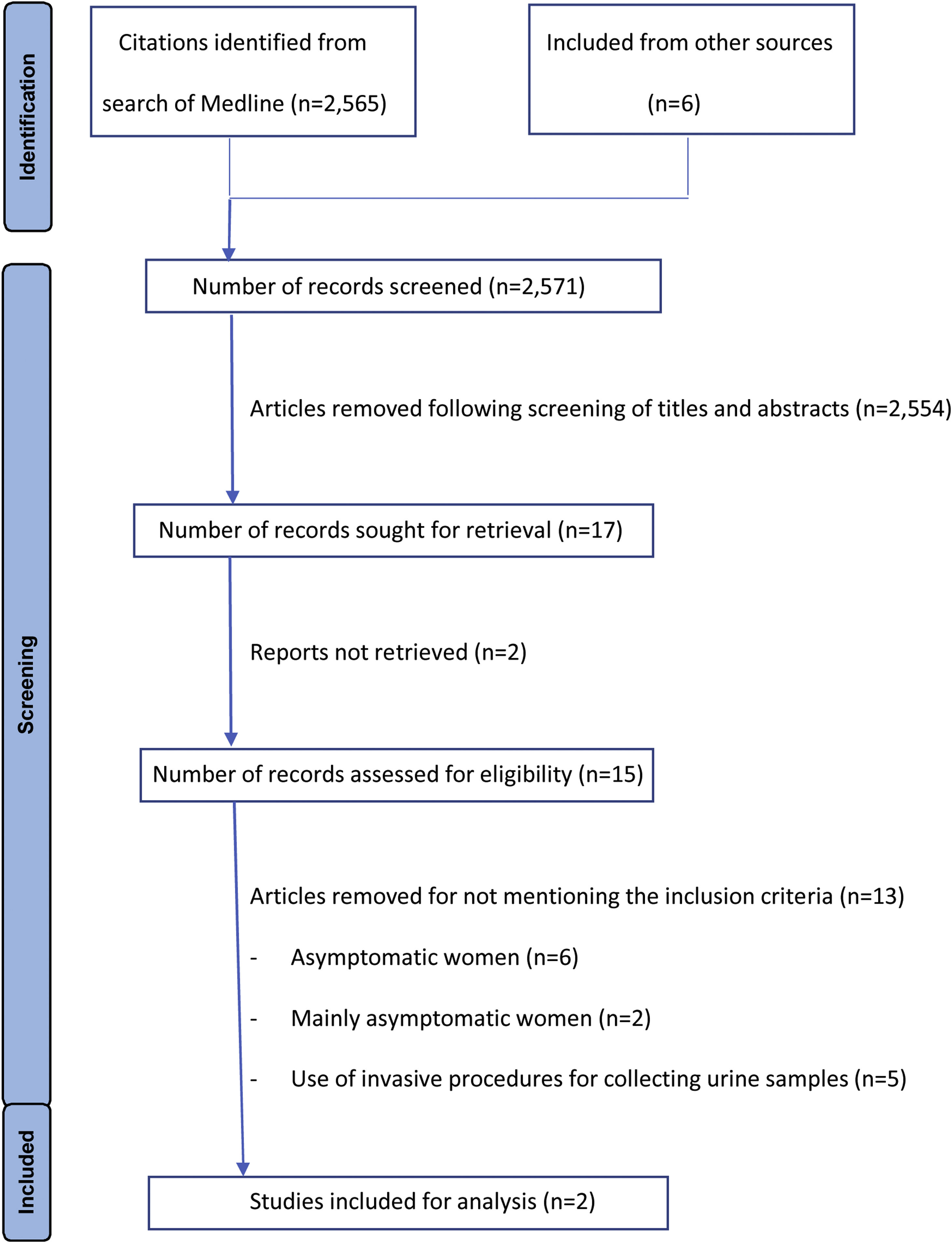
ResultsThe MEDLINE search yielded 2565 articles. After review of titles and abstracts we included 15 full text articles presenting results of studies investigating UCDs in women. A flow diagram of the literature search and review of titles, abstracts, and articles is shown in Fig. 1. Only two studies met the inclusion criteria (Table 1).7,8 A meta-analysis to explore the rate of contaminated samples was not performed as contamination was defined differently in these two studies and a narrative synthesis was therefore conducted. The list of studies excluded are described in Appendix 2, with the inclusion of asymptomatic women being the most common reason for exclusion. The quality of the studies was moderate (Appendix 3).
Characteristics of the studies included.
| Autor | Setting, country | Population | Comparison | Results |
|---|---|---|---|---|
| Collins,7 2020 | Community LUTS Clinic, UK | n=31. Age not mentioned, but mean age was 62±10 yr. | Paired samples in the same patients. Women presenting with symptoms of lower urinary tract symptoms, mainly UTI.Three groups: Peezy Midstream UCD (n=31), FVU (n=31), and MSU (n=31) | Contamination was defined as the presence of shed urothelial cells with the use of uroplakin III.The mean number of uroplakin positive cells was 0.88cells/80μl in the Peezy group, 0.91cells/80μl in the FVU specimens and 0.91cells/80μl in the MSU group (no statistically significant differences) |
| Hayward,8 2022 | General practice, UK | n=1264 women aged 18 yr. or over | RCT. Women with UTI symptoms allocated to any of these three groups: Peezy Midstream UCD (n=424), Whiz Midstream UCD (n=421) or MSU (n=419) | Contamination was defined as a mixed growth according to UK National Health Service Laboratory National Standard Operating Procedures.The proportion of contaminated samples were 26.5% with Peezy Midstream UCD, 28.2% with Whiz Midstream UCD and 29% with MSU (no statistically significant differences) |
FVU=first voided urine; LUTS=lower urinary tract symptoms; MSU=mid-stream urine; RCT=randomized clinical trial; UCD=urine collection device; UTI=urinary tract infection.
In the first study, Collins et al.7 compared three urine specimens from 31 patients with symptoms of lower urinary tract symptoms: collected with the use of FVU, MSU or Peezy Midstream UCD. Contamination was determined as the presence of shed urothelial cells with the use of uroplakin III, a transmembrane protein found exclusively in the urinary tract.9 The proportion of all the cells that were uroplakin positive did not differ among the sampling methods, which implies that contamination by extra-urinary tract cells is not influenced by the sampling technique. In a recent randomized controlled trial, 1264 women with UTI symptoms aged 18 or over were assigned to one of these three groups: Peezy Midstream UCD, Whiz Midstream UCD or MSU.8 A total of 24 patients, all of whom were allocated to EUCDs failed to collect a sample and device failures were reported by 100 out of the 395 patients assigned to Peezy (25.3%) and 35 out of the 398 participants using Whiz (8.8%). The proportion of contaminated samples was similar in the three groups of women (Table 1).
DiscussionThere is lack of studies evaluating the impact of external UCDs on the percentage of contaminated urine samples. Only two studies have evaluated the impact of these devices among women with symptoms of UTI, the population with the greatest potential for widespread benefit from the collection of urine samples. It is disappointing that so many of the studies originally identified had to be excluded, but there were clear reasons for the exclusion. The inclusion of only two studies constitutes the major limitation of this systematic review as evidence is insufficient for us to draw reliable conclusions about the impact of these devices on urine contamination. However, two main messages can be construed: 1) the rate of contaminated samples seems not to be different, or it is only slightly lower with the use of UCDs, with no statistical differences being observed regarding contamination rates among women with symptoms of suspected UTI, and 2) a non-negligible percentage of women is unable to successfully collect a urine sample with a UCD. This is also stated in studies comparing UCDs with invasive procedures for collecting urine specimens. In a recent study, High et al.10 found that 25% of women with different lower urinary tract symptoms, including painful bladder syndrome and urinary incontinence, preferred having the urine taken from transurethral catheterization rather than by midstream urine collection with the Peezy Mistream UCD. This shows how difficult the use of these external UCD might be for some women. Therefore, more studies are needed to better assess their usefulness of these devices in clinical practice.
FundingNo specific funding was received for this study.
Conflicts of interestC.L. declares having reported funds for research from Abbott Diagnostics. The other authors declared no conflicts of interest.