The incidence of prosthetic joint infection (PJI) is expected to increase in the coming years. PJI has serious consequences for patients, and high costs for the health system. The complexity of these infections makes it necessary to organize the vast quantity of information published in the last several years. The indications for the choice of a given surgical strategy and the corresponding antimicrobial therapy are specifically reviewed.
The authors selected clinically relevant questions and then reviewed the available literature in order to give recommendations according to a pre-determined level of scientific evidence. The more controversial aspects were debated, and the final composition was agreed at an ad hoc meeting. Before its final publication, the manuscript was made available online in order that all SEIMC members were able to read it and make comments and suggestions.
Se prevé un incremento de la incidencia de infección de las prótesis articulares (IPA) en los próximos años. Las IPA plantean graves consecuencias para los pacientes y un alto coste el sistema sanitario. La complejidad de estas infecciones hace que sea necesario organizar la inmensa cantidad de información publicada en los últimos años. En estas guías se revisan específicamente las indicaciones para la elección de una estrategia quirúrgica dada y el tratamiento antimicrobiano correspondiente.
Los autores seleccionaron las preguntas clínicamente relevantes y revisaron la literatura disponible con el fin de proporcionar recomendaciones de acuerdo con un grado de evidencia científica predeterminada. Los aspectos más controvertidos fueron debatidos y la redacción final se acordó en una reunión ad hoc. Antes de su publicación, el manuscrito estuvo abierto a comentarios y sugerencias de los miembros de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica.
The incidence of prosthetic joint infection (PJI) is expected to increase in the years to come. The occurrence of a PJI dramatically raises the economic costs of an arthroplasty and it is also catastrophic for the patient. The algorithm proposed by Zimmerli a decade ago represents a notable step forward in the management of these infections, and subsequent publications have confirmed its clinical usefulness. The vast quantities of data on PJI published in recent years, along with the inherent complexity of these infections, make it necessary to organize and analyze the available information.
The initiative of the present guidelines comes from the Spanish Network for the Study of Infectious Diseases (REIPI, http://reipi.org). The guideline focuses on the management of PJI by classifying all the possible therapeutic scenarios according to clinical presentation. The indications for the choice of a given surgical strategy and a particular antimicrobial therapy are specifically reviewed.
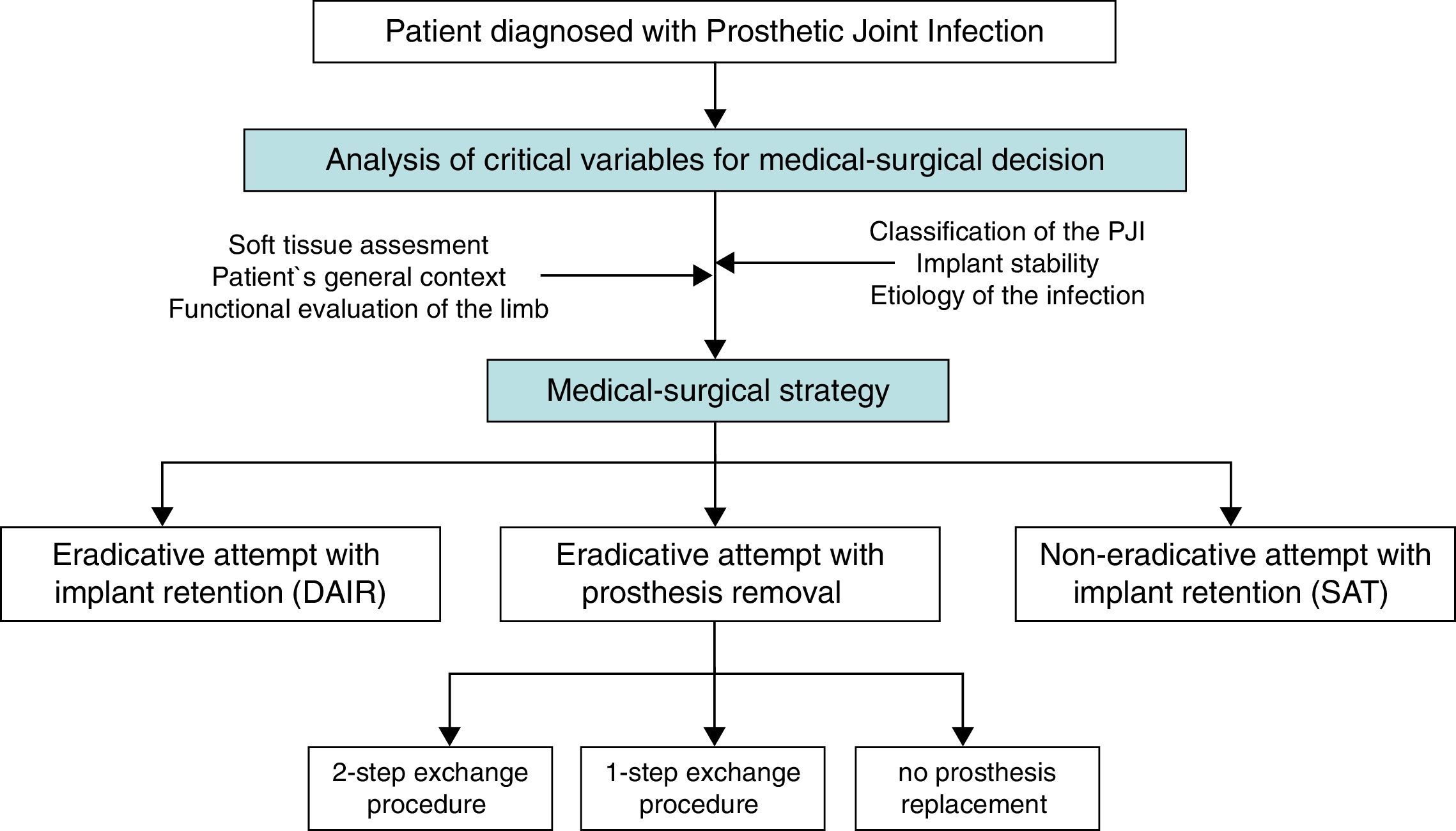
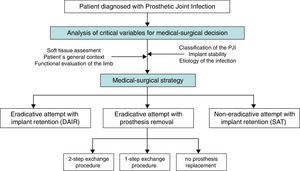
A “choice chart” was set up for the creation of these guidelines, including five possible clinical scenarios, which raised several clinical questions of interest (Fig. 1). A review of the literature published since 1970 was performed in order to answer these questions with a predetermined degree of scientific evidence.
These guidelines are addressed to professionals of orthopedic surgery, infectious disease specialists, internal medicine physicians, microbiologists, and all other health professionals responsible for the everyday management of patients with PJI. They may also be useful for other specialists who participate less frequently in the treatment of these patients, such as geriatricians, rheumatologists, physical therapy specialists, and plastic surgeons. The whole document is available in the online version.1
Initial assessment of a patient with PJIWhat are the goals of treatment?The aims of the treatment of a patient with PJI are to eradicate the infection, alleviate the pain and, at the same time, restore the joint's function. This makes PJI different from other infections in which the eradication of the infection alone may be sufficient for evaluating a given therapeutic strategy. In the case of PJI, all three goals must be considered in combination, since sometimes achieving one of these targets (i.e., eradication of the infection) may interfere with another (i.e., achieving a satisfactory functional outcome). This situation increases the complexity of the management of these patients, has a deep impact on the therapeutic decisions, and makes the interpretation of the literature difficult, since there is no standardized definition of therapeutic success.
What should the care of patients with PJI involve?- •
Due to the complexity of patients with PJI, they should be attended at multidisciplinary units.(C-III)
The main medical and surgical strategies to be considered in a patient with PJI are:
- •
Attempted eradication with implant retention and antibiotics (Debridement, antibiotics and implant retention: DAIR)
- •
Attempted eradication with implant removal and antibiotics
- ∘
With prosthesis replacement (in a 1-step or a 2-step exchange procedure).
- ∘
Without prosthesis replacement (arthrodesis or resection arthroplasty).
- ∘
- •
Implant retention and long-term suppressive antibiotics (SAT), without attempted eradication.
The decision regarding the most appropriate medical and surgical strategy for a given patient should consider features of the prosthesis, the patient's baseline condition, his/her previous functional performance, life expectancy, desires and expectations, and also the surgical risk involved. Tsukayama's and Zimmerli's classifications of PJI are both helpful for guiding medical and surgical decisions in a given patient. These classifications are based on similar criteria, which take into account pathogenic aspects, the time of infection, and the diagnostic circumstances.
When is attempted eradication with implant retention (DAIR) indicated? What are the results?- •
The best candidates for attempting eradication treatment with implant retention are those who:
- ∘
have an early post-surgical (up to three months after the placement of the prosthesis) or hematogenous infection (A-II), with a stable implant, and surrounding skin and soft tissues in good condition.
- ∘
have a short duration of symptoms (≤3 weeks) (B-II).
- ∘
can be treated with rifampin (staphylococcal infections) or fluoroquinolones (infections caused by GNB) (A-II).
- ∘
- •
Some patients who do not strictly meet the above criteria may still benefit from this strategy, but its implementation should be considered on an individualized basis, since there is a higher likelihood of failure (B-II).
- •
The prosthesis should be removed in cases of chronic PJI (A-II).
- •
A 2-step exchange procedure is recommended in patients with chronic PJI (A-II).
- •
In patients with acute PJI who are not candidates for eradication treatment with implant retention, a 2-step exchange procedure is recommended (B-II).
- •
The performance of a 1-step exchange procedure may be considered in non-immunosuppressed patients if they have good bone stock, if the prosthetic surrounding soft tissues are in good condition, and if the infection is caused by microorganisms susceptible to antibiotics with good activity against sessile (biofilm-embedded) bacteria (B-II).
- •
In patients with acute PJI in whom the removal of the prosthesis is not very complex, a 1-step exchange procedure is recommended as long as the causative microorganisms are susceptible to antibiotics with good activity against biofilm-embedded bacteria (C-III).
Suppressive antimicrobial therapy (SAT) is seen as an alternative strategy for cases of PJI in which the surgical treatment cannot be performed or will be insufficient for eradicating the infection. The following conditions need to be met for the indication of SAT:
- •
Identification of the microorganism causing the infection.
- •
Availability of oral antibiotics which are not toxic when administered over long periods of time. Possibility of a close follow-up of the patient.
In addition, it should be considered that pain due to looseness or implant instability will be not reverted by SAT.
- •
Treatment with SAT may be considered in situations in which medical and surgical strategies are unlikely to cure the patient, and non-toxic long-term antimicrobials are available (B-II).
- •
Treatment with SAT is not indicated in acute PJI managed early, with appropriate debridement and optimized antimicrobial therapy (E-II).
- •
Surgical debridement must be performed promptly by an expert surgical team, with the patient in the best possible condition (C-III).
- •
The surgical approach must be performed by open arthrotomy. Arthroscopy should only be considered in selected cases, and performed by expert surgeons (A-II).
- •
The surgical debridement must be aggressive, methodical and exhaustive.
- ∘
If feasible, the removable components of the prosthesis should be exchanged (B-II).
- ∘
Copious irrigation (≥9L of saline) is recommended with no additives, performed by a low-pressure system (C-III).
- ∘
See Table 1
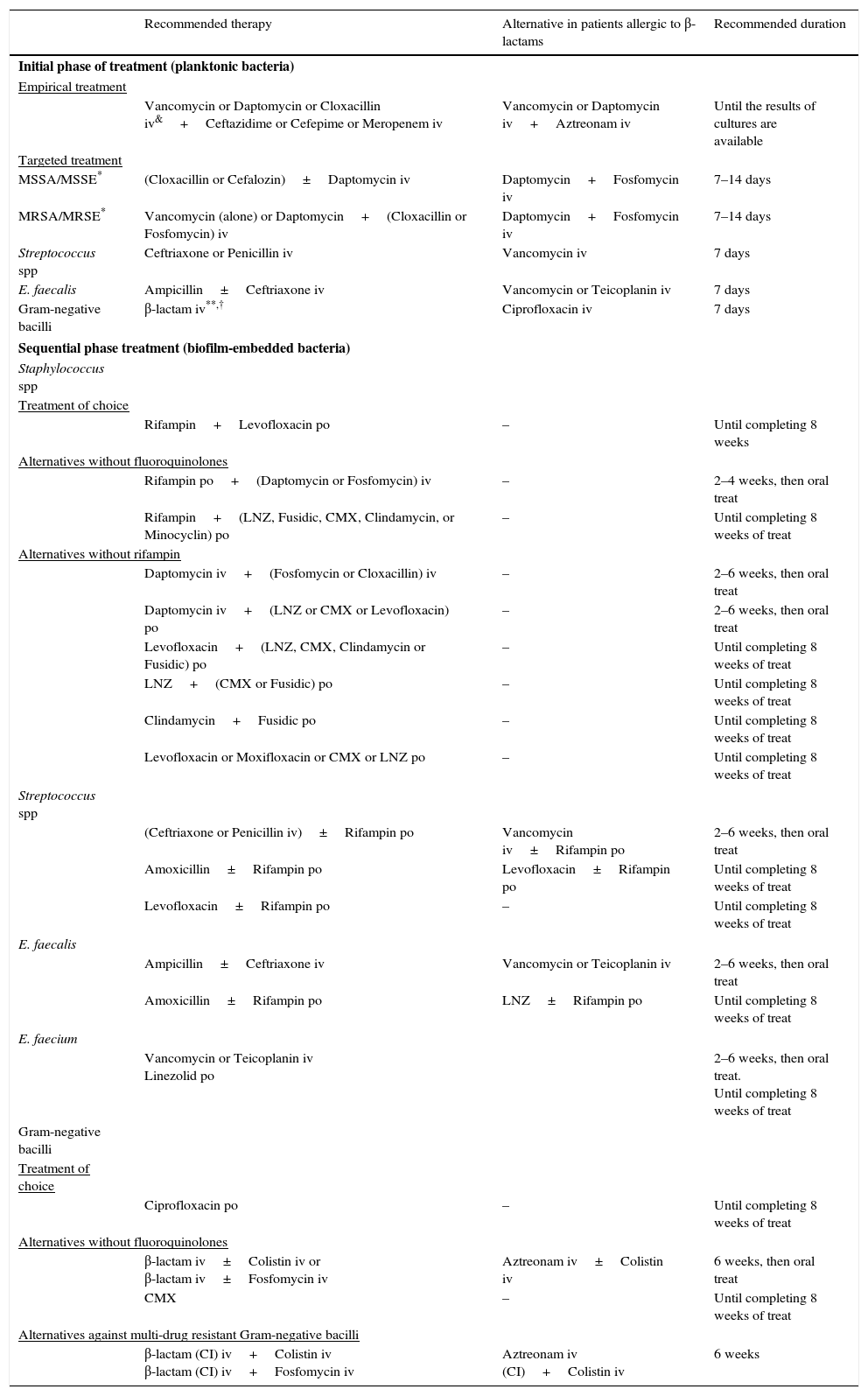
Empirical and targeted antimicrobial therapy in the eradicative attempt of management with implant retention.
| Recommended therapy | Alternative in patients allergic to β-lactams | Recommended duration | |
|---|---|---|---|
| Initial phase of treatment (planktonic bacteria) | |||
| Empirical treatment | |||
| Vancomycin or Daptomycin or Cloxacillin iv&+Ceftazidime or Cefepime or Meropenem iv | Vancomycin or Daptomycin iv+Aztreonam iv | Until the results of cultures are available | |
| Targeted treatment | |||
| MSSA/MSSE* | (Cloxacillin or Cefalozin)±Daptomycin iv | Daptomycin+Fosfomycin iv | 7–14 days |
| MRSA/MRSE* | Vancomycin (alone) or Daptomycin+(Cloxacillin or Fosfomycin) iv | Daptomycin+Fosfomycin iv | 7–14 days |
| Streptococcus spp | Ceftriaxone or Penicillin iv | Vancomycin iv | 7 days |
| E. faecalis | Ampicillin±Ceftriaxone iv | Vancomycin or Teicoplanin iv | 7 days |
| Gram-negative bacilli | β-lactam iv**,† | Ciprofloxacin iv | 7 days |
| Sequential phase treatment (biofilm-embedded bacteria) | |||
| Staphylococcus spp | |||
| Treatment of choice | |||
| Rifampin+Levofloxacin po | – | Until completing 8 weeks | |
| Alternatives without fluoroquinolones | |||
| Rifampin po+(Daptomycin or Fosfomycin) iv | – | 2–4 weeks, then oral treat | |
| Rifampin+(LNZ, Fusidic, CMX, Clindamycin, or Minocyclin) po | – | Until completing 8 weeks of treat | |
| Alternatives without rifampin | |||
| Daptomycin iv+(Fosfomycin or Cloxacillin) iv | – | 2–6 weeks, then oral treat | |
| Daptomycin iv+(LNZ or CMX or Levofloxacin) po | – | 2–6 weeks, then oral treat | |
| Levofloxacin+(LNZ, CMX, Clindamycin or Fusidic) po | – | Until completing 8 weeks of treat | |
| LNZ+(CMX or Fusidic) po | – | Until completing 8 weeks of treat | |
| Clindamycin+Fusidic po | – | Until completing 8 weeks of treat | |
| Levofloxacin or Moxifloxacin or CMX or LNZ po | – | Until completing 8 weeks of treat | |
| Streptococcus spp | |||
| (Ceftriaxone or Penicillin iv)±Rifampin po | Vancomycin iv±Rifampin po | 2–6 weeks, then oral treat | |
| Amoxicillin±Rifampin po | Levofloxacin±Rifampin po | Until completing 8 weeks of treat | |
| Levofloxacin±Rifampin po | – | Until completing 8 weeks of treat | |
| E. faecalis | |||
| Ampicillin±Ceftriaxone iv | Vancomycin or Teicoplanin iv | 2–6 weeks, then oral treat | |
| Amoxicillin±Rifampin po | LNZ±Rifampin po | Until completing 8 weeks of treat | |
| E. faecium | |||
| Vancomycin or Teicoplanin iv Linezolid po | 2–6 weeks, then oral treat. Until completing 8 weeks of treat | ||
| Gram-negative bacilli | |||
| Treatment of choice | |||
| Ciprofloxacin po | – | Until completing 8 weeks of treat | |
| Alternatives without fluoroquinolones | |||
| β-lactam iv±Colistin iv or β-lactam iv±Fosfomycin iv | Aztreonam iv±Colistin iv | 6 weeks, then oral treat | |
| CMX | – | Until completing 8 weeks of treat | |
| Alternatives against multi-drug resistant Gram-negative bacilli | |||
| β-lactam (CI) iv+Colistin iv β-lactam (CI) iv+Fosfomycin iv | Aztreonam iv (CI)+Colistin iv | 6 weeks | |
Abbreviations: x: during; MRSA: methicillin-resistant S. aureus; MSSA: methicillin-susceptible S. aureus; MRSE: methicillin-resistant S. epidermidis (and other coagulase-negative staphylococci); MSSE: methicillin-susceptible S. epidermidis (and other coagulase-negative staphylococci). CMX: co-trimoxazole; Fusidic: fusidic acid; LNZ: linezolid; CI: continuous infusion; iv: intravenous treatment; po: per os (oral route); treat: treatment.
Recommended doses (assuming normal renal function): Cloxacillin, 2g/4h iv; Vancomycin, 1g/12h iv; Daptomycin, 8–10mg/kg/24h iv; Ceftazidime, 2g/8h iv; Aztreonam, 2g/8h iv; Cefepime, 2g/8–12h iv; Meropenem 1–2g/8h iv; Ertapenem, 1g/24h iv; Ceftriaxone 2g/24h; Ampicillin: 2g/6h iv; Amoxicillin, 1g/8h po; Rifampin, 600mg/24h po; Levofloxacin, 500–750mg/24h po; Moxifloxacin, 400mg/24h po; Ciprofloxacin, 400mg/12h iv or 750–1000mg/12h po; Linezolid, 600mg/12h po; Fusidic Acid, 500mg/8h po; Fosfomycin, 2g/6h iv; Colistin, 6–9 millions IU/d (8–12h) iv; Co-trimoxazole 800/160mg/8h po; Clindamycin, 600mg/6–8h po; Minocycline, 200mg/d po.
The choice of a particular anti-staphylococcal agent may be conditioned by the presence of bloodstream infection, especially in hematogenous infections.
The choice of a particular β-lactam agent against Gram-negative bacilli depends on the species and mechanisms of resistance: ceftriaxone is the treatment recommended for Enterobacteriaceae, except if they produce chromosomal β-lactamases (i.e., AMPc) or plasmidic extended-spectrum β -lactamases (ESBL); in these cases, the use of ertapenem will be preferred; in infections caused by P. aeruginosa, an anti-pseudomonal β-lactam is recommended.
Foreign-body infections are characterized by the presence of sessile (biofilm-embedded) bacteria in a stationary phase of growth. However, it is also important to consider planktonic bacteria (in a logarithmic phase of growth) in these infections, especially when they are acute. An optimized initial antibiotic treatment with good activity against rapidly-growing planktonic bacteria should be provided. Once the most inflammatory component of the infection and the initial bacterial inoculum have been reduced, the treatment can focus on the biofilm-embedded bacteria.
- •
After surgical debridement, antibiotics with good activity against rapidly-growing planktonic bacteria should be provided, ideally based on β-lactams, lipopeptides, or glycopeptides (B-III).
- •
This initial treatment must be administered intravenously for at least 7 days before switching to an optimized antimicrobial therapy focused on the treatment of biofilm-embedded bacteria (C-III).
- •
Initial treatment (antibiotics against planktonic bacteria)
- ∘
Methicillin-susceptible strains: cloxacillin (or cefazolin) (B-II), or cloxacillin+daptomycin (C-III).
- ∘
Methicillin-resistant strains: daptomycin+cloxacillin, or daptomycin+fosfomycin(C-III), or vancomycin (B-II).
- ∘
- •
Subsequent treatment (against biofilm-embedded bacteria)
- ∘
Treatment of choice: rifampin+levofloxacin (A-II).
- ∘
If fluoroquinolones cannot be used: combinations of rifampin with co-trimoxazol (B-II), linezolid (B-II), clindamycin (B-II), fusidic acid (B-II), or daptomycin (B-III).
- ∘
If rifampin cannot be used: combinations of daptomycin with fosfomycin (B-III), cloxacillin (B-III), linezolid (B-III), co-trimoxazol (C-III), or levofloxacin (C-III); or combinations of 2 oral antibiotics or monotherapy with levofloxacin (B-III), or moxifloxacin (B-III), co-trimoxazol (BIII), or linezolid (B-III).
- ∘
- •
For initial treatment (planktonic phase): penicillin or ceftriaxone (B-II).
- •
Subsequent treatment (biofilm-embedded bacteria): penicillin or ceftriaxone (B-II), followed by amoxicillin (BII), either in combination with rifampin or not (B-III); alternatively, levofloxacin (B-III) either in combination with rifampin or not (B-III), or monotherapy with clindamycin or linezolid in the case of allergy to fluoroquinolones (C-III).
- •
The treatment of choice is ampicillin, followed by oral amoxicillin (B-II).
- •
It can be administered in combination with ceftriaxone (B-III) or rifampin (B-III).
- •
Teicoplanin or linezolid are possible alternatives (C-III).
- •
For initial treatment (planktonic phase): a β-lactam (a 3rd-generation cephalosporin for Enterobacteriaceae, a carbapenem for ESBL or AMP β-lactamase producing GNB, and an anti-pseudomonal β-lactam for P. aeruginosa) (B-III).
- •
Subsequent treatment (biofilm-embedded bacteria):
- ∘
Treatment of choice: a fluoroquinolone (ciprofloxacin) (A-II).
- ∘
If fluoroquinolones cannot be used (due to resistance, toxicity, etc.): continue treatment with a β-lactam (B-III) combined or not with colistin (B-III) or fosfomycin (C-III), or monotherapy with co-trimoxazole (C-III).
- ∘
- •
If possible, the use of antibiotics prior to a valid sampling (i.e. joint aspirate, and/or intraoperative cultures) should be avoided (B-III).
- •
The antimicrobial treatment must be active against the most prevalent microorganisms. The need for antibiotic activity against multi-drug resistant microorganisms must be considered in accordance with the patient's clinical and epidemiological context (C-III).
- •
If antibiotics have been administered prior to the sampling and they are considered as potentially responsible for the absence of microbiological diagnosis, the antimicrobial spectrum of this treatment should be considered when choosing the new antibiotic regime (C-III).
- •
For acute staphylococcal PJI managed with rifampin and levofloxacin, an 8-week schedule of treatment after debridement appears sufficient for most patients (B-I).
- •
For PJI caused by other microorganisms treated with antibiotics with good activity against biofilm-embedded bacteria (i.e. ciprofloxacin for PJI caused by GNB), 8 weeks is also a reasonable duration (B-III).
- •
In other clinical scenarios, the most appropriate duration of treatment remains uncertain. A variable period between 8 and 12 weeks may be adequate (B-III).
- •
Monitoring of CRP during the follow-up is advisable; the persistence of high values is suggestive of treatment failure (B-III), but its total normalization must not be a condition for deciding the end of therapy (B-II).
- •
During antimicrobial therapy, a close follow up of observance and potential adverse events of the treatment is recommended, performed by a clinician with expertise in antibiotics (C-III).
- •
During the first 6 months after the end of a treatment aiming at eradication, patients must be followed up closely (B-III).
- •
The frequency of follow-up visits may decrease afterwards. Follow-up should last at least one year (B-III).
- •
The two-step exchange procedure should include a targeted intravenous antimicrobial treatment for 4 to 6 weeks (A-II), or 1–2 weeks of intravenous antibiotics followed by oral antimicrobials with good bioavailability for a total duration of 6 weeks (B-II).
- •
In chronic PJI caused by CNS, “universal” anti-staphylococcal antimicrobial therapy (i.e. glycopeptides, daptomycin, or linezolid) may be considered after the first-step surgery (prosthesis removal), because this carries a lower rate of positive cultures during the second-step surgery (re-implantation) (C-III).
- •
Shortening the systemic antimicrobial treatment could be considered for cases of PJI due to low-virulent microorganisms, such as CNS or Propionibacterium acnes, as long as the first-step surgery has included a thorough and exhaustive debridement of the joint, and a cement spacer loaded with antibiotics active against the microorganism responsible for the infection has been used (B-II).
- •
When samples taken during the second-step surgery yield a microorganism, a new 4–6 week course of antibiotics is recommended (B-II).
- •
At present, it is not clear whether rifampin should be administered to treat staphylococcal infection managed with a two-step exchange procedure.
- ∘
The indication of rifampin in a chronic non-inflammatory infection should be based on the thoroughness of the surgical debridement (C-III).
- ∘
Rifampin is recommended in cases with a significant inflammatory presentation, especially those caused by S. aureus(C-III).
- ∘
- •
Antibiotic-loaded spacers are recommended in the two-step exchange procedure (B-II).
- •
The dose of local antibiotic ranges between 0.5 and 4g of vancomycin, and 0.25 and 4.8g of gentamycin or tobramycin (per every 40g of acrylic cement) (C-III).
- •
The use of combined local antibiotics (vancomycin-gentamicin) is recommended until further evidence specifically addressing this topic is available (C-III).
- •
In PJI caused by multi-drug resistant microorganisms, spacers may be still used as long as they are loaded with antibiotics active against these microorganisms (C-III).
- •
In the two-step exchange procedure, an antibiotic-free period of 2–8 weeks and clinical stability before the second-step surgery is recommended (C-III).
- •
The monitoring of ESR and/or CRP is recommended. The persistence of values above the normal range does not necessarily indicate the persistence of the infection, and sore-implantation should not be delayed (B-II). However, significant changes in these serum markers may imply the persistence of the infection or a superinfection (C-III).
- •
Sampling of tissues and the cement spacer during the second-step surgery of a two-step exchange procedure is recommended in order to guarantee the sterility of the surgical site where the new prosthesis is to be placed (B-II).
- •
Culture of the joint aspirate before the second-step surgery is not systematically recommended, although it may be of some use when the clinical and analytical evaluation of the patient suggests poor evolution, or in difficult-to-treat episodes caused by multi-drug resistant microorganisms or fungi (C-II).
- •
Cultures of samples taken during the second-step surgery may be considered as positive if ≥1 or ≥2 of them yield a microorganism, depending on its pathogenicity (C-III).
- •
Wide-spectrum antibiotic prophylaxis including nosocomial microorganisms that may potentially cause superinfection of the new prosthesis is recommended for the second-step surgery of a 2-step exchange procedure (C-III).
- •
“Preemptive treatment” including microorganisms that could be isolated during the second-step surgery (usually multi-drug resistant SNC) is recommended: vancomycin (or another glycopeptide or lipopeptide) during the first 5 days after re-implantation or until confirmation that the samples taken during the second-step surgery yield no microorganisms (C-III).
Recommendations
- •
Beginning an antimicrobial therapy 3–5 days prior to the 1-step exchange procedure is recommended if the etiological diagnosis has already been made, especially in infections caused by S. aureus or GNB (C-II).
- •
Regardless of the decision regarding when to start antibiotics, an appropriate antimicrobial prophylaxis throughout the procedure must be guaranteed (A-I).
- •
If no antimicrobial therapy has been initiated before the procedure, it should be delayed until the intraoperative sampling has been performed (C-III).
- •
A minimum of 7 days of intravenous antibiotics with activity against the microorganisms causing the infection is recommended (dosage summarized in Table 1), followed by oral antibiotics for a total of 4–8 weeks (B-II).
- •
If it has been decided to use a cemented prosthesis, a local antibiotic with activity against the microorganism causing the infection is recommended. If the etiology is unknown at the moment of the exchange procedure, the combination of vancomycin plus gentamycin is recommended (C-III).
- •
In the case of PIOC (Tsukayama's classification) an antimicrobial treatment of 4–6 weeks is recommended. There is no need for further surgery. The same protocol is followed as in cases of PJI managed with a 1-step exchange procedure (B-III).
- •
For cases in which the infected prosthesis is not to be replaced after its removal, the same antibiotics as those used for DAIR may be administered (see table) (B-II).
- •
In these cases, the length of therapy may be shortened to 4 to 6 weeks (C-III).
Implant retention and long-term suppressive antibiotics (SAT) without attempted eradication.
- •
A surgical debridement before beginning SAT is recommended, if feasible (C-III).
- •
Obtaining a valid sample for culture before starting SAT is particularly important (C-III).
- •
For the choice of the specific antibiotic for SAT, the antimicrobial susceptibility of the microorganism causing the infection, the safety of the drug and the observance of the treatment must be considered. Except for the initial stages of SAT, these aspects must prevail over the optimization of the antimicrobial treatment (C-III).
- •
Except for some particular cases, the use of combinations (and therefore the use of rifampin) is not recommended (D-III).
- •
In cases undergoing surgical debridement, an initial intravenous treatment for at least 7 days is recommended. Nevertheless, prolonged intravenous treatment is not necessary when deciding on SAT management (C-III).
- •
If it is necessary to stop or change the antibiotics due to the occurrence of adverse events, long periods without antibiotics are not recommended (D-III).
- •
The prescription and control of a SAT must be performed by an expert in antimicrobial therapy, who will periodically follow up the clinical evolution of the infection and assess the possible occurrence of adverse events (B-III).
- •
The use of linezolid is discouraged in SAT due to high risk of toxicity, which limits its prolonged administration (E-I).
- •
The use of β-lactams, or low doses of co-trimoxazole, is recommended. Alternatively, other antimicrobials such as minocycline or clindamycin may be administered (C-III).
JA has served as speaker for and has received fees for advisory boards from Pfizer and Novartis.
JC has served as speaker for Astellas, AstraZeneca, MSD, Novartis and Angellini, and has received fees for advisory boards from Astellas, Pfizer, AstraZeneca and MSD.
JE has recived fees for lectures from Laboratorios Leti and support for attending conferences from Pfizer, bioMérieux, Alere, Laboratorios Leti and Novartis.
JPH has served as speaker for MSD, Astellas, Novartis, Pfizer, and Astra Zeneca and also has received fees for advisory boards from MSD, Astellas, Novartis, Pfizer, Angelini, Basilea and Astra Zeneca.
CP has received fees from Pfizer, MSD, Astellas, Novartis, Zambon, Salvat, and Mefasa-pharma.
DRP has received lecture fees, travel support for attending meetings and fees for advisory boards from Novartis, Astellas, Merck and Pfizer.
AS has received fees as speaker from Pfizer, Novartis, MSD and Astellas.
NB declares having received honoraria from Pfizer, Novartis, MSD, Astellas and Astra-Zeneca for development of educational presentations, consultancy tasks and/or for the payment of travel/accommodation for scientific purposes.
The rest of the authors declare no conflict of interests.
We thank Michael Maudsley for reviewing the English version, and Juan Manuel García-Lechuz for his critical review of the document.
J.L.-T holds a clinical research contract “Sara Borrell” (CD14/00176) from the Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness.
Supported by Plan Nacional de I+D+i and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015) – co-financed by European Development Regional Fund “A way toachieve Europe” ERDF.