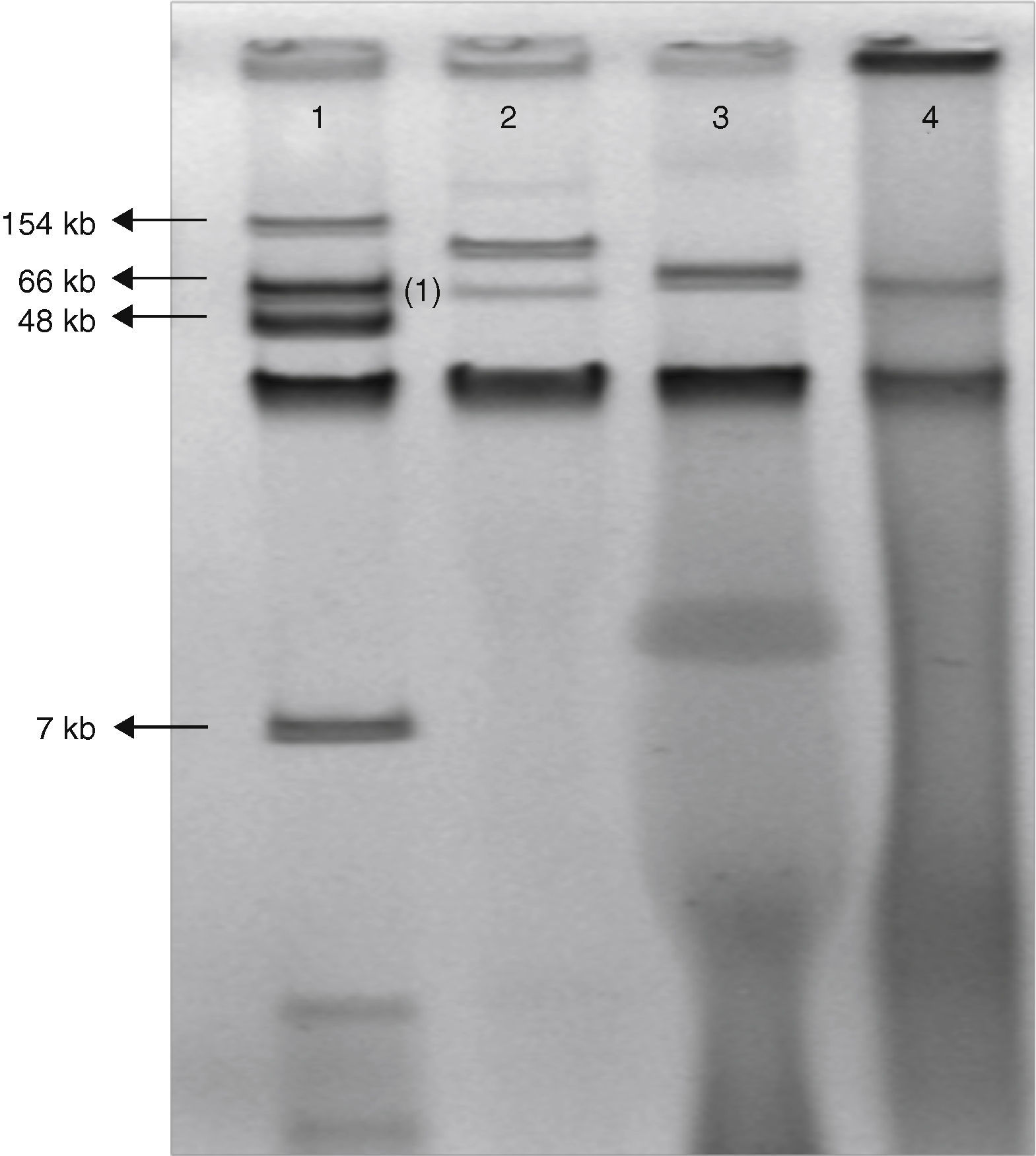
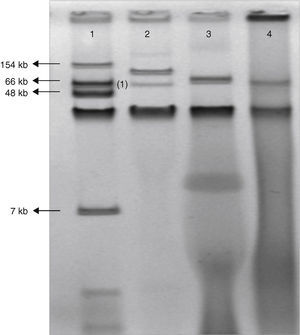
The class D beta-lactamase OXA-48 confers resistance to penicillins and decreased susceptibility to carbapenems, but has weak or no activity against expanded-spectrum cephalosporins. This enzyme was first detected in 2001 from a Klebsiella pneumoniae isolate from Istanbul, Turkey.1 Since then, OXA-48-producing Enterobacteriaceae have been mainly described throughout the Mediterranean area and Africa as a cause of both sporadic cases and outbreaks, mainly, coexpressing extended spectrum betalactamases (ESBL). On 27 June 2011, a 65-year-old man was hospitalized at the Coronary Care Unit of the University Hospital Virgen Macarena, Seville, Spain, because of an ischemic coronary episode. A surgical coronary revascularization procedure was performed on the 14th day of admission. Two days after surgery the patient was feverish; empirical therapy with piperacillin/tazobactam (PTZ) was started. Seven days after surgery, purulent exudate was noted in the sternotomy wound, where E. aerogenes was recovered and from blood cultures on the 2nd and 10th day after surgery. PTZ was switched to meropenem when the susceptibility tests of the first blood isolate was shown to be resistant to PTZ. The infection was resolved and the patient was discharged one month later. Barrier precautions and intensive room cleaning were implemented, and no more clinical cases were detected. All isolates were found to be resistant by using a commercial panel (Wider, Francisco Soria Melguizo, Spain) to penicillins, betalactams plus betalactamase inhibitors combinations, cefuroxime, and susceptible to the third generation cephalosporins, quinolones and imipenem MIC value of all isolates was 2mg/L. The modified Hodge test was positive for all of 3 isolates recovered from exudate and both blood cultures and all isolates showed identical XbaI PFGE pattern. PCR experiments followed by sequencing identified only blaOXA-48 gene. Conjugation experiments were performed using E. coli J53 azide resistant like recipient cell. Transconjugants were selected on Mac Conkey agar supplemented with ertapenem 0.25mg/L and azide 150mg/L. Carbapenems susceptibility of the clinical isolates and transconjugants was performed by microdilution broth. Both clinical and transconjugants isolates exhibited MICs of imipenem=2mg/L, ertapenem=4mg/L, and meropenem=4mg/L. Plasmid DNA extraction by using the Kieser technique revealed a plasmid of approximately 70kb (Fig. 1). Identification of the incompatibility group of these plasmids failed using a PCR-based replicon typing method. The amplification of the phage replication protein P (RepP)2 gene was carried out and positive results were obtained. All these features together suggest a close relationship with the plasmid found spreading in the Mediterranean Sea countries and in Western Europe, which possesses an Inc L/M scaffold.3,4 To date, this determinant has been only associated to single 60–70kb IncL/M-type plasmid backbone.5 The genetic environment of the blaOXA-48 gene was determined by PCR using primers matching the insertion sequence IS1999 and primers matching the upstream and downstream region of the blaOXA-48 gene to determine the transposon type. These specific primers were designed as follows: IS1999-middle, 5′-CCTTAGAGGCCAGCATCAAGCTG-3′; OXA-48-5ext, 5′-GCAGGCATTCCGATAATCGAT; OXA-48-3ext, 5′-GGATATGCCCACATCGGATGGT. Sequencing results confirmed the presence of the Tn1999.2 transposon: an IS1R insertion sequence truncated the transposase gene of IS1999 as previously described.6
Gel electrophoresis of plasmid extraction by Kieser technique from Escherichia coli 50192 (lane 1), Klebsiella pneumoniae 11978 (lane 2), Enterobacter aerogenes clinical isolate (lane 3) and E. coli J53 harboring conjugant plasmid pOXA-48 (lane 4).
We describe the first E. aerogenes isolate carrying OXA-48 in absence of ESBLs. An E. cloacae isolate had been previously identified in a patient in Spain during an outbreak of OXA-48-producing K. pneumonie.7 The susceptibility pattern of OXA-48 alone confers a higher difficulty in the detection of OXA-48 producers, because the MICs of carbapenems can remain in the susceptible or intermediate range. Previous cases of infection or colonization by OXA-48 producers had not been detected in our hospital. Previous relation with endemic countries or a prior history of hospitalization could not be established in our case. Both the low MICs of carbapenems and the lack of epidemiological link show the current challenge of detecting and implementing effective control measures to avoid the dissemination of plasmids harboring OXA-48.
Supported by Ministerio de Economía y Competitividad, Instituto de Salud Carlos III – co-financed by European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015).