The first Ebola virus infected patient outside Africa was diagnosed and treated at Alcorcón Foundation University Teaching Hospital (AFUTH). We describe the integrated management strategy (medical, occupational health, preventive and public health) applied to the case.
MethodsDescriptive study of health-care management of an unexpected case of Ebola virus disease (EVD) at AFUTH treated on 6 October 2014. We describe the clinical evolution of the patient while he was attended at the Emergency Department, the drawing-up process of the action protocol, the process of training of hospital staff, the administrative management for transferring the patient to the referral centre, and the measures implemented for cleaning, disinfection and management of waste. Qualitative variables are expressed as percentages.
ResultsOur centre designed and updated, from May to October, five versions of the acting and care protocol for patients with EVD. The protocol was in force at the AFUTH when a nursing assistant was attended on 6 October 2014. All preventive, diagnostic and therapeutic measures outlined in the protocol were applied and 206 professionals had received training and information about care procedures with a suspect case.
ConclusionHealth-care management of an unexpected case of EVD was adequate and there was no secondary cases in our staff as a result. All resources available should be used to fight EVD.
El Hospital Universitario Fundación Alcorcón (HUFA) diagnosticó y atendió al primer caso diagnosticado de EVE fuera de África. Nuestro objetivo es describir la atención sanitaria (médica, preventiva y de salud pública) dada a la paciente.
MétodosEstudio descriptivo de la gestión de un caso inesperado de enfermedad por virus de Ébola, en el HUFA, atendido el día seis de octubre de 2014. Se hace una descripción de la evolución clínica de la paciente mientras estuvo atendida en urgencias del HUFA, del proceso de diseño del protocolo de actuación, del proceso de formación e información al personal del hospital, de la gestión administrativa para su traslado al centro de referencia y del seguimiento de las medidas de limpieza, desinfección y gestión de los residuos producidos. Las variables cualitativas se describen con porcentajes.
ResultadosNuestro centro diseñó y actualizó, desde el mes de mayo hasta octubre, cinco versiones del protocolo de atención a pacientes con EVE. El protocolo estaba en vigor en el HUFA y 206 profesionales habían recibido formación e información sobre el mismo y los procedimientos de atención a un caso sospechoso. Se atendió el 6 de octubre a una auxiliar de enfermería a la que se aplicaron todas las medidas preventivas, diagnósticas y terapéuticas contempladas en el protocolo.
ConclusiónLa atención prestada al caso inesperado de EVE fue adecuada y no hubo ningún caso secundario en nuestro personal como consecuencia de su atención. Hay que utilizar todos los recursos a nuestro alcance para combatir la EVE.
Although Ebola virus disease (EVD) has been known since 1976,1–5 until now cases had occurred exclusively in Africa and in outbreaks that were largely confined to specific areas. The current EVD outbreak began in Guinea-Conakry in December 2013,6 and the number of cases has been rising continuously ever since. Almost all the cases recorded to date have occurred in areas with few resources for combating them and have affected the native population, but international aid and co-operation through humanitarian and religious organisations has nevertheless led to cases occurring among non-native patients, who have then been repatriated to their countries of origin. Despite the fact that these patients have been treated at hospitals equipped with modern technology and adequate means to treat EVD, the end result is that disease transmission has become a real risk to the health personnel involved, with the ensuing risk of cases appearing outside the disease's initial reservoir in Africa.7–9
In its first week, the clinical course of EVD is marked by non-specific fever and severe headaches, and is accompanied by gastrointestinal symptoms (abdominal pain, nausea, vomiting and diarrhoea); in the second week, haemorrhagic symptoms and sepsis may appear. If patients survive the second week, they usually improve and recover.10
While overall Ebola virus mortality stands at 40–88%, that of the Zaire ebolavirus species implicated in the current outbreaks is 78%, a figure higher than that of any other EVD-producing strain.4,10,11
The Alcorcón Foundation University Teaching Hospital (AFUTH) diagnosed and attended the first patient to acquire the disease outside Africa. This study sought to outline the integrated management strategy (medical, preventive and public health) applied to this case at our health centre, with a description of the care given to the patient, the steps leading to the drawing-up of our action protocol, and the measures implemented after the patient's evacuation and transfer to the referral hospital.
Materials and methodsThis was conceived as a descriptive study of health-care management of an unexpected case of Ebola virus disease. The situation was deemed to be an outbreak in accordance with the definition of an outbreak as the appearance of any isolated case of the disease in a hitherto unaffected area.12 This EVD case was attended at the AFUTH on 6 October 2014.
The AFUTH drew up an action protocol in the event of the possible appearance of a case of EVD at our health centre. The protocol was an adaptation of the suspected EVD-case action protocol issued by the Spanish Ministry of Health, Social Services & Equality (MH) (Ministerio de Sanidad, Servicios Sociales e Igualdad). The MH published its first protocol on 7 April 2014 and the AFUTH made its first adaptation on 5 May 2014. Since then, both the MH and AFUTH have been adapting their respective protocols in response to the course taken by the outbreaks in Africa and the trends in the international epidemiological situation. The AFUTH protocol was designed by a multidisciplinary team whose members were drawn from the preventive medicine, prevention, emergencies, intensive care, clinical analysis, microbiology, radiology, paediatrics, internal medicine, nursing, communication and quality departments, and management in charge of the nursing, economic-financial, health-care and administration sections.
The health authorities designated the Carlos III Hospital as the referral centre for the care of possible cases of EVD in the Madrid Region (Comunidad de Madrid). The strategy decided upon was to centralise care of any suspected or confirmed case of EVD at this hospital. This strategy changed in the month of August, when it was decided that any hospital in the Madrid Region should thenceforth attend a suspected case, until the disease was either diagnosed or ruled out.
The action designed in the AFUTH's first action protocol (May 2014) consisted of defining a care circuit for suspected cases, by isolating them with standard, contact and droplet precautions until arrangements could be made for their transfer to the referral hospital. From August onwards, the action strategy changed in order to comply with MH guidelines for defining the isolation circuit, care of suspected cases and patient diagnostic procedure.
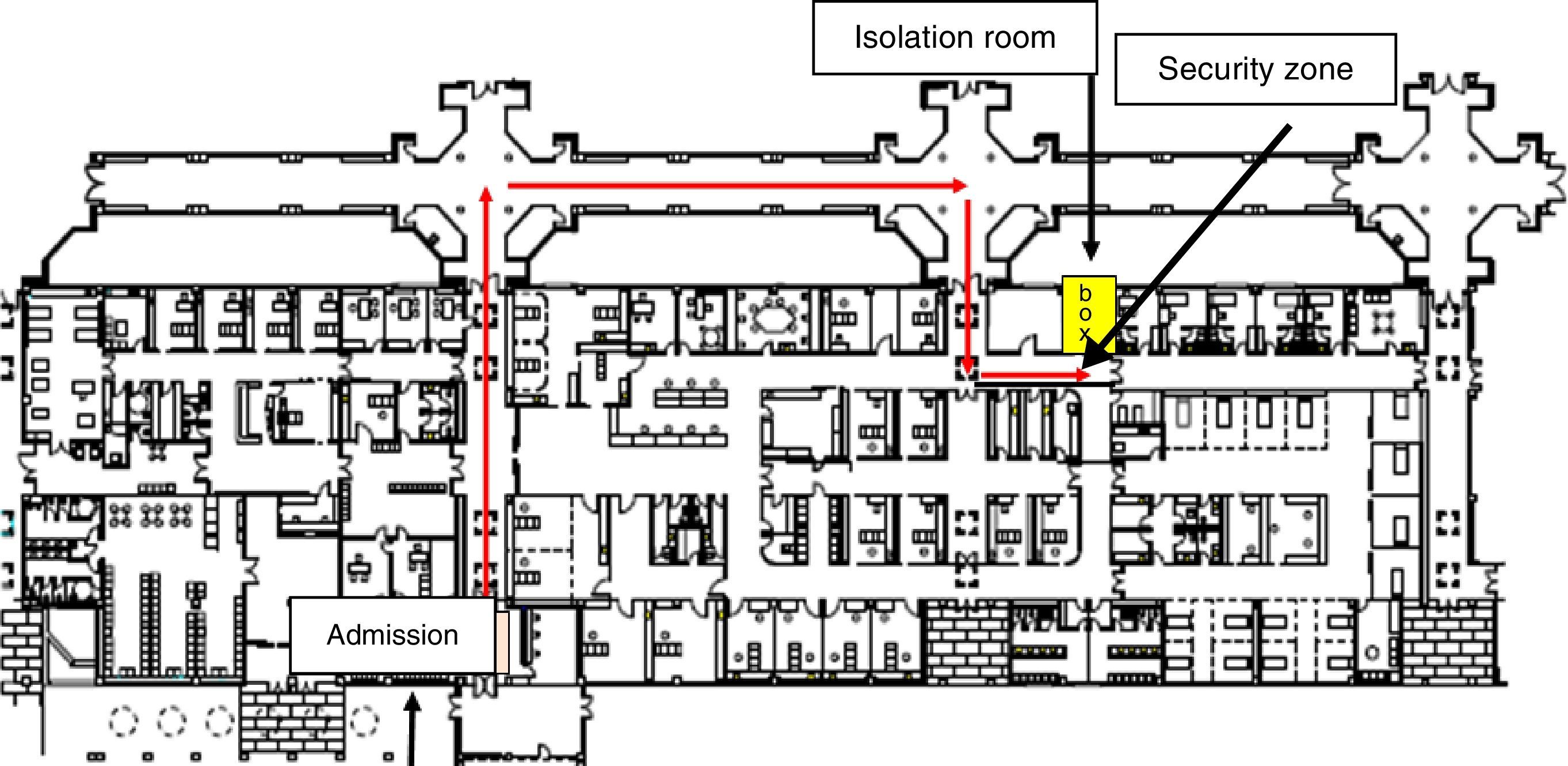
The AFUTH protocol made provision for the following preventive measures: (1) definition of a single isolation room with private bathroom in the emergency ward (Fig. 1); (2) case-definition and implementation of a system for early diagnosis of suspected EVD among emergency admissions, by asking patients about fever, compatible clinical signs and symptoms, and the epidemiological criterion of arriving from a country with confirmed cases or contact with a patient diagnosed with or suspected of having EVD (Table 1); (3) design of specific circuit for transferring suspected cases from emergency admissions to the room and thence to the exterior in the event of evacuation; (4) recommendation and implementation of contact and droplet isolation measures; (5) demarcation of a security zone around the isolation room, by sealing off corridors and doors, and using screens, notices and signs to restrict circulation to specified members of staff regarded as indispensable for attending to the patient; (6) definition of the dispatch of diagnostic samples to the National Microbiology Centre in line with the MH protocol, and installation of a laboratory mini-analyser (Alere Healthcare®) for point-of-care testing (POCT), with a specific circuit outside the routine laboratory circuit for handling urgent analytical samples of suspected cases where, in the physician's opinion, the patient's clinical course might render this essential and impossible to defer until the latter's transfer to the referral centre; (7) description of the measures for cleaning/decontaminating the room, and segregating and disposing of biomedical waste; and, (8) plan for training and instructing health-care workers involved in the care of patients with suspected or confirmed EVD. We designed a training plan for emergency staff (physicians, paediatricians, nurses, nursing assistants and hospital porters), laboratory personnel (laboratory physicians and technicians) and staff of the diagnostic imaging unit (diagnostic radiographers).
Ebola virus disease case-definition.
| Clinical criteria | Fever >38.6° and any of the following:Severe headache;Vomiting, diarrhoea and abdominal pain;Any unexplained haemorrhagic manifestation; orMulti-organ failure. |
| Laboratory criteria | Detection of Ebola virus nucleic acid in a clinical specimen |
| Epidemiological criteria | At least one of the following in the 21 days preceding symptom onset:Having been in an area with community transmission;aHaving had contact with a probable or confirmed EVD case; or,Contact with a probable or confirmed case's bodily fluids or biological samples. |
Room cleaning/decontamination measures consisted of cleaning up bodily fluids (secretions, vomit, diarrhoea, blood, etc.) with disposable wipes, cleaning surfaces stained with bodily fluids with disposable wipes, pouring bleach onto bathroom surfaces after use, and sterilising the room with vaporised hydrogen peroxide after use. The wipes used were impregnated with a quaternary ammonium disinfectant (didecyldimethylammonium chloride). All waste products generated were segregated in special biomedical waste containers which were then sealed, externally disinfected with quaternary ammonium disinfectant and disposed of for sterilisation.
The plan for the training and instruction of the relevant health-care workers consisted of a first stage of briefings about the epidemiology of the disease, type of transmission, prevention measures and personal protective equipment (PPE) to be used, depending on the risk as determined by the patient's clinical condition. Basic equipment was defined as gloves, surgical mask, fluid-resistant or impermeable gown, and face shield or goggles, and its use was indicated in evaluation at admission, transfer to the room, and initial clinical examination and recording the medical history of patients not presenting with symptoms of advanced disease. Complete equipment was defined as double gloving, surgical mask, fluid-resistant or impermeable gown, face shield or goggles and disposable shoe covers, and its use was indicated in the clinical care of patients presenting with symptoms of advanced disease. In the event of having to use complete equipment, the option was given of using a full-body coverall with a one-piece overhood (Tyvek® classic Xpert) instead of a fluid-resistant or impermeable gown. In the case of aerosol production (intubation, endoscopy), the use of an FFP2 (filtering facepiece) mask was indicated.
During this first stage, the donning of PPE was theoretically explained, and a practical demonstration was then given by the trainer. Signs were placed at the entrance to and inside the isolation room containing information about isolation and prevention measures.
A second stage of practical training was planned, specifically designed to supervise and show staff how to put on and remove PPE.
The following were described: the patient's clinical progress while being attended at the AFUTH emergency ward; the process of drawing up the action protocol; the hospital staff training and instruction process; the administrative management of the patient's transfer to the referral hospital; and the follow-up of waste cleaning, disinfection and management measures. Qualitative variables were expressed as percentages.
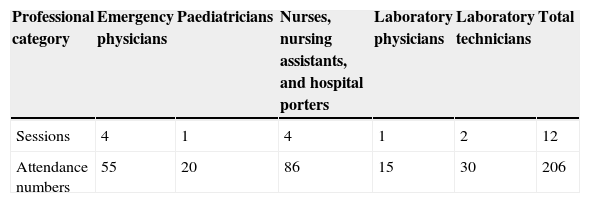
ResultsBetween May and October 2014, our health centre designed and updated five versions of the EVD patient care protocol. As regards the AFUTH staff training and instruction envisaged in the protocol, by October the emergency and laboratory personnel had received the training shown in Table 2. The training completed at the date of the patient's admission was that comprising the first stage: the second, more specific training stage had not yet been implemented.
At 7:00h on 6 October 2014, our health centre attended to a nursing assistant who presented with a clinical profile compatible with the EVD definition. She herself had cared for a patient with EVD who had died on 25 September 2014, a fact she reported on admission to the hospital emergency ward. Although this patient did not meet the clinical criteria (she had no fever), she did fulfil the epidemiological criteria for being deemed a suspected case, in that she was in the 21-day incubation period following contact with a subject diagnosed with EVD. The above action protocol was immediately implemented and the patient was transferred to a single room with private bathroom, purpose-defined for such a possible case. After reviewing the case in consultation with public health officers from the Madrid Regional Health Authority, a blood sample was taken to confirm or rule out EVD and malaria. The sample was sent to the Majadahonda National Microbiology Centre at 10:00h, and confirmation of its having tested positive was received later that same day at 18:30h. EVD-positive status was detected using quantitative polymerase chain reaction (PCR) assay. The patient was attended at all times by an emergency physician and nurse who had the support and advice of an intensive care specialist from the Critical Care Unit, and the patient was kept clinically stable until she could be evacuated to the referral centre, i.e., the Carlos III Hospital. Once confirmation was received that the test had proved positive, arrangements were made, with the help of the public health officers, for the patient to be transferred to the referral centre, with the transfer finally taking place after the patient had been in the emergency ward for 18h.
The physician and nurse who attended the patient had to put on the PPE placed at their disposal as often as was required by the disease's clinical course, a total of thirteen times. PPE was donned in obedience to the clinically assessed level of risk or the health professional's considered opinion, and consisted of basic equipment during the initial evaluation and examination of the patient, and full-body equipment when evidence of advanced disease appeared, i.e., vomiting, diarrhoea and haemorrhaging. In the latter case, the professionals used a coverall.
Following the patient's evacuation, the room was cleaned and disinfected/decontaminated in accordance with the action-protocol guidelines, which in fact coincide with those contained in the hospital's general cleaning and disinfection protocol. In addition, the room was sterilised with vaporised hydrogen peroxide. Control of the effectiveness of sterilisation was assessed using physical, chemical and biological controls. The complete process of cleaning, disinfection and control assessment took three days, and the room was once again available for use on the fourth day following care of the EVD patient.
All waste products produced while caring for the patient were segregated in accordance with the hospital's general protocol for special biomedical waste management. As a result, fifty-one special biomedical waste containers were generated, sealed and disinfected externally, and then eliminated by means of the standard hospital elimination procedure for sterilisation of this type of waste.
Patient care was undertaken by a total of eleven members of the health-care staff, who were then themselves followed-up as risk contacts for the maximum EVD incubation period of twenty-one days. The public health authorities classified eight as high-risk for not having used double gloving or fluid-resistant or impermeable gowns, and were placed in quarantine at the Carlos III Hospital for surveillance in isolation. The remaining three were followed up on an ambulatory basis, with active monitoring of symptomatology and temperature. This active follow-up consisted of self-monitoring and recording of body temperature twice daily, and supervision and evaluation of the reported temperature by physicians in the AFUTH Occupational Health Department. Contacts were required to be reachable by telephone on a 24-h basis and to call the hospital twice daily. In addition, the staff had instructions to call the physicians in the event of any doubt or suspicion of compatible clinical signs and symptoms. In no case did any of the contacts develop the disease.
The patient admitted to the referral centre, the Carlos III Hospital, made good progress, recovered and was finally discharged on 5 November 2014.
DiscussionThe appearance of EVD cases outside Africa has posed a challenge to public health and health-care services of the countries in which such cases have started to arise,9,13,14 and due to the fact that this is a new situation – outside the niche of the African countries in which the current outbreaks are evolving – there is a dearth of information for tackling these new cases.15 This study describes the experience of integrated management of clinical, microbiological and preventive care, public health and occupational risks, in the first case of Ebola virus disease diagnosed outside Africa.
In May 2014, our hospital drew up a protocol for the care of a possible case of EVD, which was regularly updated in line with outbreak developments in Africa and the availability of new data. The protocol's fundamental aim was to provide appropriate care for possible cases and ensure the health and wellbeing of health-care workers and the general population.
The application of the protocol to the care of the EVD case attended by us proved satisfactory. The patient was immediately identified as a suspected case on admission to emergencies, even though she did not fulfil the clinical criterion of fever stipulated in the protocol. She was directly transferred to the purpose-defined isolation room, and the recommended contact and droplet isolation measures were then implemented.16,17 Staff members had PPE at their disposal at all times as recommended in the literature, and fears voiced about attending to possible cases led the hospital to purchase and supply health professionals with one-piece coveralls to meet the expressed need for security. This is a point of pivotal importance in the care of such patients: not only must emergency services be on the alert and ready to conduct rapid examination and diagnosis of suspected EVD patients, but the definition of appropriate measures to ensure protection and prevention and the availability of PPE are equally crucial.18–21 The personnel at our health centre had not received specific training in the donning and doffing of PPE because the case's unexpected appearance meant that the training plan could not be fully implemented. This plan has now been implemented however, and at the date of writing, staff belonging to all areas involved in the care of these patients have received specific training and instruction in how to put on and remove PPE.
In view of the fact that the patient did not present with fever at the date of the medical visit, this criterion has been slightly nuanced in the latest version of our and the MH protocols. Among contacts, the criterion has been lowered to 37.7°C, and stress is now laid on inquiring about the taking of antipyretics or immunosuppressive agents that might mask the fever.
Mention should also be made of the management of the collection and processing of possible analytical samples. A specific laboratory circuit with a POCT auto-analyser was designed to process individual samples outside the laboratory's general routine procedure. This was done to make provision for a case where analytical specimens might have to be requested if the patient's clinical situation required this, at a point in time when the EVD diagnostic results were still pending. As it happened, there was no need to use this equipment because the only clinical samples requested were those of Ebola virus and malaria, and these were processed by the National Microbiology Centre.
The cleaning and disinfection/decontamination of the room were performed in accordance with the routine hospital protocol, with the appropriate PPE being recommended to all cleaning staff. The AFUTH engaged the services of a specialised cleaning firm for this task, and the room was thoroughly cleaned and disinfected, and subsequently sterilised with vaporised oxygen peroxide. The room was used normally after verification of standard physical and chemical and negative biological controls, and was pronounced operational and once again ready for use four days after care of the EVD patient.
Eleven members of the hospital staff who were directly involved in patient care were followed-up as risk contacts for the maximum 21-day EVD incubation period. Eight of these were classified by the public health authorities as “high-risk” and were quarantined at the Carlos III Hospital for surveillance in isolation. The remaining three were followed up on an ambulatory basis with active surveillance of symptomatology and temperature. While the reason for this decision can be understood from the stance of trying to protect both health professionals and the general population from contagion in the case of such a severe disease, this approach is not scientifically supported.22 It has been clearly shown that an asymptomatic person is not contagious, and that a patient, albeit infected, is not contagious in the asymptomatic stage. Indeed, it is known that fever precedes the state of contagiousness, and that even at the date of onset of fever there is still no transmissibility: a positive PCR assay is detected two or three days after the appearance of fever. Patient follow-up under surveillance must be ensured by using a strict monitoring protocol, keeping a record of the temperature and, in the event of a rise in the patient's temperature and onset of fever, reporting this to the health authorities so that the pertinent measures can be taken for isolation, diagnosis and treatment.18,22
The current EVD outbreak,23 which has been under way for almost one year now,24 is a challenge that must be tackled, and all the resources at our disposal must be used for the purpose, including prevention, diagnosis and treatment measures which form part of a uniform and coherent public health strategy.25
It can be concluded that, within the possibilities and resources available, the care given to the unexpected cases of EVD attended at the Alcorcón Foundation University Teaching Hospital was adequate. The patient was attended, diagnosed, treated and transferred to the referral centre in accordance with the recommendations in the literature, and there were no secondary cases among our staff arising out of the care given to the patient.
Conflict of interestThe authors declare that no competing financial interests exist.
Acevedo García, Manuel; Algora Weber, Alejandro; Alonso Punter, Juan Carlos; Casas Losada, Maria Luisa; Castilla Castellano, Virgilio; De los Frailes Sanz, José Luis; Delgado-Iribarren, Alberto; Díaz Cuasante, Ana Isabel; Echávarri Olavarría Fernando; Espino Hernández, Maria del Mar; Garrido Martín, Modoaldo; Lorenzo Martínez, Susana; Losa Garcia, Juan Emilio; Meléndez Agudín, Victoria; Noguera Quijada, Carmen; Pérez Vega, Francisco José; Prieto Alaguero, Pilar; Rodríguez Caravaca, Gil; Romero Mari, Elena; Salmerón Beliz, Octavio; Sánchez Vidal, Begoña; Timermans del Olmo, Rafael; Torres Rivera, Carmen; Trapero García, Miguel Ángel and Velasco Arribas, María.