The aim of this study was to analyze the distribution of human papillomavirus (HPV) genotypes in cytologically abnormal cervical samples from 106 women living in a region of the north of Spain.
MethodsCytological classification was reported according to the 2001 Bethesda System and HPV genotyping was performed by Roche Linear Array.
ResultsThe overall HPV prevalence was 69.8% with 30 different HPV genotypes detected. The prevalence of HR (high-risk) HPV types and pHR (probable high-risk) HPV types in positive samples was 94.3%, 78.1% and 100% in patients with ASCUS, LSIL and HSIL/CC, respectively, with no significant differences. The most frequent type was the HPV 16, present in 29.7% of all positive samples, followed by HPV 51 (17.5%), HPV 53 and 42 (16%), HPV 52 (12%), HPV 39 (10.8%), HPV 18 and 58 (9.4%) and HPV 66 (8.1%). No significant differences in the percentage of any HPV genotype with the grade of the cytological lesion were detected. The prevalence of HPV co-infection was 58.1% of HPV positive.
ConclusionsThis study confirms the high prevalence of high-risk genotypes in women with abnormal cytology living in our geographical area. This information may be useful for the formulation of algorithms for patient management according to the different risks associated with specific high-risk genotypes.
El objetivo de este estudio es analizar la distribución de genotipos del virus del papiloma humano (HPV) en citologías anormales de cérvix de 106 mujeres residentes en una región del norte de España.
MétodosLa clasificación de las citologías se realizó mediante el sistema Bethesda 2001 y el genotipado del HPV por el sistema Linear Array de Roche.
ResultadosLa prevalencia del HPV fue del 69,8% y se detectaron 30 genotipos distintos de HPV. La prevalencia de genotipos de alto riesgo y probable alto riesgo en las muestras positivas fue del 94,3, 78,1 y 100% en pacientes con ASCUS, LSIL y HSIL/CC, respectivamente, sin alcanzar significación estadística. El genotipo 16 fue el más frecuente (29,7% de todas las muestras positivas), seguido por los genotipos HPV 51 (17.5%), HPV 53 y 42 (16%), HPV 52 (12%), HPV 39 (10,8%), HPV 18 y 58 (9,4%), y HPV 66 (8,1%). Para ningún genotipo se observó diferencia en la prevalencia dependiendo del grado de lesión citológica. La prevalencia de coinfección fue del 58,1% de los casos positivos.
ConclusiónEste estudio confirma la alta prevalencia de los genotipos de alto riesgo en mujeres con citología anormal residentes en nuestra área geográfica. La información obtenida puede ser útil en la elaboración de algoritmos para la gestión de pacientes en función del riesgo asociado a genotipos específicos de alto riesgo.
Genital human papillomavirus (HPV) infection is considered to be one of the most common sexually transmitted infections (STIs) worldwide and has been associated with cervical cancer, the second most common cancer in women with ages ranging from 15 to 44 years.1 The worldwide HPV prevalence in women with normal cytology is estimated to be 10.4%.2 The prevalence of infection varies greatly, being dependent on the target population, age distribution and the severity of the disease.3,4 In Spain, the prevalence in women with normal cytology ranges from 3% to 8.3%,5–7 depending on the geographical area; these values are similar to those in the surrounding countries, such as Italy, with prevalence data of around 7%.8 The prevalence is higher in Latin American women living in Spain (21% in Argentinians and 27% in Colombians).6 According to a recent study,7 prevalence is even higher in several high-risk groups such as commercial sex workers and women in prison, being 29.9% in Spaniards, 23.1% in migrant Africans and 32.8% in migrant Latin Americans belonging to these risk groups.
More than 100 genotypes of HPV have currently been identified based on the nucleotide sequences comparison of specific regions, such as coding regions of E6, E7 and L1 proteins.9 In Spain, HPV types 16, 31, 52, 68, 51, 53, 18, 33, 45, 58 and 66 were most common in women from the general population.7 A retrospective cross-sectional worldwide study including 10,575 cases of invasive cervical cancer from 38 countries in five continents has shown that HPV types 16, 18 and 45 were the three most common types in each histological group (squamous cell carcinoma, adenocarcinoma and adenosquamous cell carcinoma). Invasive cervical cancers associated with types 16, 18 and 45, irrespective of the histological type, are diagnosed at a much younger age (<50 years), and at an average of 4 years earlier than are those caused by other high-risk HPV types.10 The HPV prevalence increases as the grade of the cytological lesion increases.11 Knowledge of the distribution of HPV types in patients with cervical abnormalities in a specific area could contribute to providing information regarding regional variations in the HPV type spectrum, to evaluating the level of cross-protection between related HPV types, and to designing second generation multivalent vaccines targeting the HPV types that predominate in different geographical areas.
The main aim of this study was to assess the prevalence of single HPV and multiple HPV in a cohort of 106 women with cytological abnormalities and its association with squamous intra-epithelial lesions (SIL). HPV genotyping was performed on cell suspensions from the cervix by Roche Linear Array which differentiates 37 HPV genotypes. The HPV genotype distribution has been compared to that obtained in other Spanish regions.
MethodsSample collection and processingFrom January 2009 to January 2010, 106 consecutive women attending the Gynaecology Outpatients Clinic of Santiago Hospital in Vitoria (capital of the Basque Country, Spain) were prospectively enrolled. These women were referred for HPV testing due to an abnormal Papanicolaou smear. The population studied was considered as being at a low epidemiological risk, and all cases came from screening programs (secondary screening) implemented by their local health services. A single cytological sample per woman was studied. The women were stratified by age groups: 18–25 years (n=28), 26–35 (n=35), 36–45 (n=27) and >45 (n=16). Cytological classification was reported according to the 2001 Bethesda System,12 scoring as atypical squamous cells of undetermined significance (ASCUS), low grade squamous intra-epithelial lesion (LSIL), high-grade intra-epithelial lesion and cervical carcinoma (HSIL/CC).
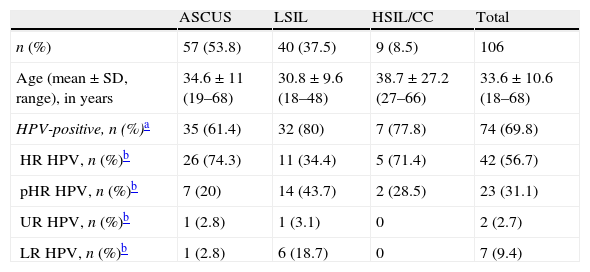
The mean age of the 106 women included in the study was 33.6±10.6 years (range 18–68). Fifty-seven (53.8%) patients had an ASCUS lesion, 40 (37.5%) an LSIL, 8 (7.5%) an HSIL and 1 patient had cervical cancer (Table 1).
HPV prevalence and genotypes in women according to cytological status.
| ASCUS | LSIL | HSIL/CC | Total | |
| n (%) | 57 (53.8) | 40 (37.5) | 9 (8.5) | 106 |
| Age (mean±SD, range), in years | 34.6±11 (19–68) | 30.8±9.6 (18–48) | 38.7±27.2 (27–66) | 33.6±10.6 (18–68) |
| HPV-positive, n (%)a | 35 (61.4) | 32 (80) | 7 (77.8) | 74 (69.8) |
| HR HPV, n (%)b | 26 (74.3) | 11 (34.4) | 5 (71.4) | 42 (56.7) |
| pHR HPV, n (%)b | 7 (20) | 14 (43.7) | 2 (28.5) | 23 (31.1) |
| UR HPV, n (%)b | 1 (2.8) | 1 (3.1) | 0 | 2 (2.7) |
| LR HPV, n (%)b | 1 (2.8) | 6 (18.7) | 0 | 7 (9.4) |
Percentage respect to HPV-positives. When more than one genotype was identified in the same sample, only the HPV genotype with the higher risk was included. ASCUS (atypical squamous cells of undetermined significance), LSIL (low grade squamous intra-epithelial lesion), HSIL (high-grade intra-epithelial lesion) and CC (cervical cancer). HR HPV (high-risk types), pHR HPV (probable high-risk types), UR HPV (undetermined-risk types) and LR HPV (low-risk types).
Cervical cells samples were obtained with an endocervical cytobrush, and placed into 20ml of PreservCyt Solution (Cytyc Corporation, USA). A ThinPrep slide (Cytyc Corporation, USA) was prepared from the cytology specimen and this was used for HPV testing by Linear Array HPV Genotyping Test (Roche Diagnostics, USA). One aliquot was removed from each 20ml PreservCyt vial before cytology analysis with a ThinPrep slide. For isolation of nucleic acid, 850μl of material was used with an AmpliLute Liquid Media Extraction kit (Roche) and was performed on the automated COBAS AmpliPrep (Roche Diagnostics, USA) assay system according to the manufacturer's protocol. Nucleic acid was resuspended in a final volume of 85μl; 50μl was used for PCR analysis.
The Linear Array HPV Genotyping Test (Roche Diagnostics, USA) employs biotinylated primers (PGMY) to define a sequence of nucleotides within the polymorphic L1 region of the HPV genome that is approximately 450 base pairs long. A pool of HPV primers is used to amplify HPV DNA from 37 HPV genotypes (types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 56, 57, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 and CP6108). An additional primer pair targets the human β-globin gene as a control for cell adequacy; extraction and amplification were also used. Amplification was performed following the manufacturer's instructions. The resulting PCR product was reverse hybridized to genotype-specific probes immobilized as parallel lines on a nitrocellulose strip. The hybridization reaction and detection were carried out with the automated ProfiBlot T48 (Tecan, Switzerland) according to the manufacturer's instructions. The Linear Array HPV Genotyping strips were read visually by comparing the pattern of blue lines with the Reference Guide and the results were evaluated by two of the authors (A.C and J.L.B.). Thirty-seven HPV genotypes can be identified simultaneously in a single hybridization step. An unambiguous, continuous band was judged to indicate that amplicons had hybridized to complementary sequences of the probes bound to strips and was considered a positive result. For this analysis, we considered types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 69, 73 and 82 to be high-risk types (HR HPV). Types 26, 53, 66 are considered to be probable high-risk types (pHR HPV) and types 34, 57, 62, 71, 83, 84, 85 and 89 as undetermined-risk types. The rest of the genotypes are considered to be low-risk types.
Statistical analysisFor statistical analysis we used the WINPEPI Computer Programs for Epidemiologists (Abramson, J.H. Epidemiologic Perspectives and Innovations 2011, version 11.3). Contingency tables were used to assess the degree of association between variables and Pearson's chi-squared test was performed to determine the level of statistical significance. Statistical significance was defined as p<0.05.
ResultsHPV DNA was detected in 74 out of the 106 women studied (69.8%). The prevalence increased with the increasing grade of the cytological lesion (Table 1), although it was not significant.
The prevalence of HR HPV types and pHR HPV types in positive samples was 94.3%, 78.1% and 100% in patients with ASCUS, LSIL and HSIL/CC, respectively. No significant differences were detected.
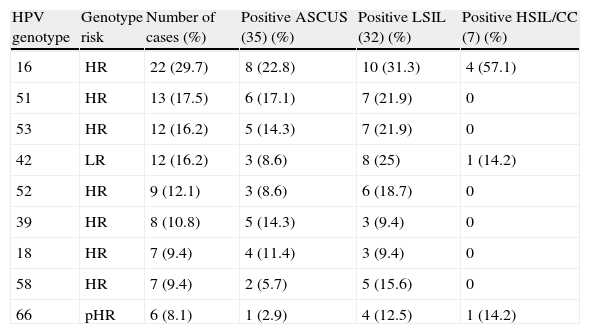
Table 2 shows the distribution of the most prevalent HPV genotypes in women included in the study. Thirty HPV genotypes were detected. The most frequent type was the HPV 16, present in 29.7% of all positive samples, followed by HPV 51 (17.5%), HPV 53 and 42 (16%), HPV 52 (12%), HPV 39 (10.8%), HPV 18 and 58 (9.4%) and HPV 66 (8.1%). No significant differences in the percentage of any HPV genotype with the grade of the cytological lesion were detected.
Distribution of most prevalent HPV genotypes in women with abnormal cervical cytology.
| HPV genotype | Genotype risk | Number of cases (%) | Positive ASCUS (35) (%) | Positive LSIL (32) (%) | Positive HSIL/CC (7) (%) |
| 16 | HR | 22 (29.7) | 8 (22.8) | 10 (31.3) | 4 (57.1) |
| 51 | HR | 13 (17.5) | 6 (17.1) | 7 (21.9) | 0 |
| 53 | HR | 12 (16.2) | 5 (14.3) | 7 (21.9) | 0 |
| 42 | LR | 12 (16.2) | 3 (8.6) | 8 (25) | 1 (14.2) |
| 52 | HR | 9 (12.1) | 3 (8.6) | 6 (18.7) | 0 |
| 39 | HR | 8 (10.8) | 5 (14.3) | 3 (9.4) | 0 |
| 18 | HR | 7 (9.4) | 4 (11.4) | 3 (9.4) | 0 |
| 58 | HR | 7 (9.4) | 2 (5.7) | 5 (15.6) | 0 |
| 66 | pHR | 6 (8.1) | 1 (2.9) | 4 (12.5) | 1 (14.2) |
ASCUS (atypical squamous cells of undetermined significance), LSIL (low grade squamous intra-epithelial lesion), HSIL (high-grade intra-epithelial lesion) and CC (cervical cancer). HR (high-risk types), pHR (probable high-risk types) and LR (low-risk types).
On the one hand, HPV 18 was detected in 11.4% of positive samples with ASCUS and 9.4% with LSIL. On the other hand, HPV 45 (n=2) was detected in 5.7% of the positive samples in patients with ASCUS.
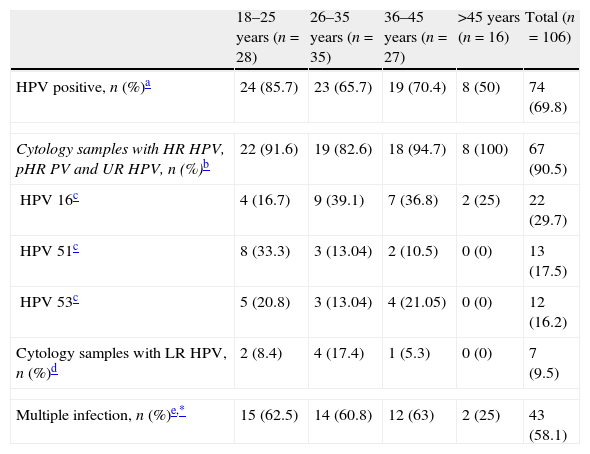
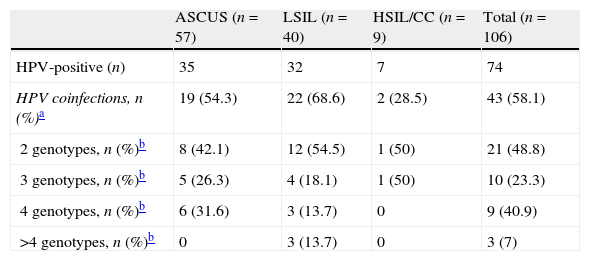
When the data were stratified by age (Table 3), women aged 18–25 years had the highest prevalence of HPV (85.7%), compared to 50% in women aged >45 years. Irrespective of the age, the HR HPV types were the most frequent (Table 3). Table 4 shows the HPV co-infection by cytological lesion type. The prevalence of HPV co-infections was 58.1% (43 out 74 positive samples) and was peaked for women with LSIL (68.6%, 22/32). At least one HR HPV genotype was detected in 93% of the samples with multiple infections.
HPV prevalence and HPV co-infections by age distribution.
| 18–25 years (n=28) | 26–35 years (n=35) | 36–45 years (n=27) | >45 years (n=16) | Total (n=106) | |
| HPV positive, n (%)a | 24 (85.7) | 23 (65.7) | 19 (70.4) | 8 (50) | 74 (69.8) |
| Cytology samples with HR HPV, pHR PV and UR HPV, n (%)b | 22 (91.6) | 19 (82.6) | 18 (94.7) | 8 (100) | 67 (90.5) |
| HPV 16c | 4 (16.7) | 9 (39.1) | 7 (36.8) | 2 (25) | 22 (29.7) |
| HPV 51c | 8 (33.3) | 3 (13.04) | 2 (10.5) | 0 (0) | 13 (17.5) |
| HPV 53c | 5 (20.8) | 3 (13.04) | 4 (21.05) | 0 (0) | 12 (16.2) |
| Cytology samples with LR HPV, n (%)d | 2 (8.4) | 4 (17.4) | 1 (5.3) | 0 (0) | 7 (9.5) |
| Multiple infection, n (%)e,* | 15 (62.5) | 14 (60.8) | 12 (63) | 2 (25) | 43 (58.1) |
Prevalence of HPV coinfections per cytological lesion type.
| ASCUS (n=57) | LSIL (n=40) | HSIL/CC (n=9) | Total (n=106) | |
| HPV-positive (n) | 35 | 32 | 7 | 74 |
| HPV coinfections, n (%)a | 19 (54.3) | 22 (68.6) | 2 (28.5) | 43 (58.1) |
| 2 genotypes, n (%)b | 8 (42.1) | 12 (54.5) | 1 (50) | 21 (48.8) |
| 3 genotypes, n (%)b | 5 (26.3) | 4 (18.1) | 1 (50) | 10 (23.3) |
| 4 genotypes, n (%)b | 6 (31.6) | 3 (13.7) | 0 | 9 (40.9) |
| >4 genotypes, n (%)b | 0 | 3 (13.7) | 0 | 3 (7) |
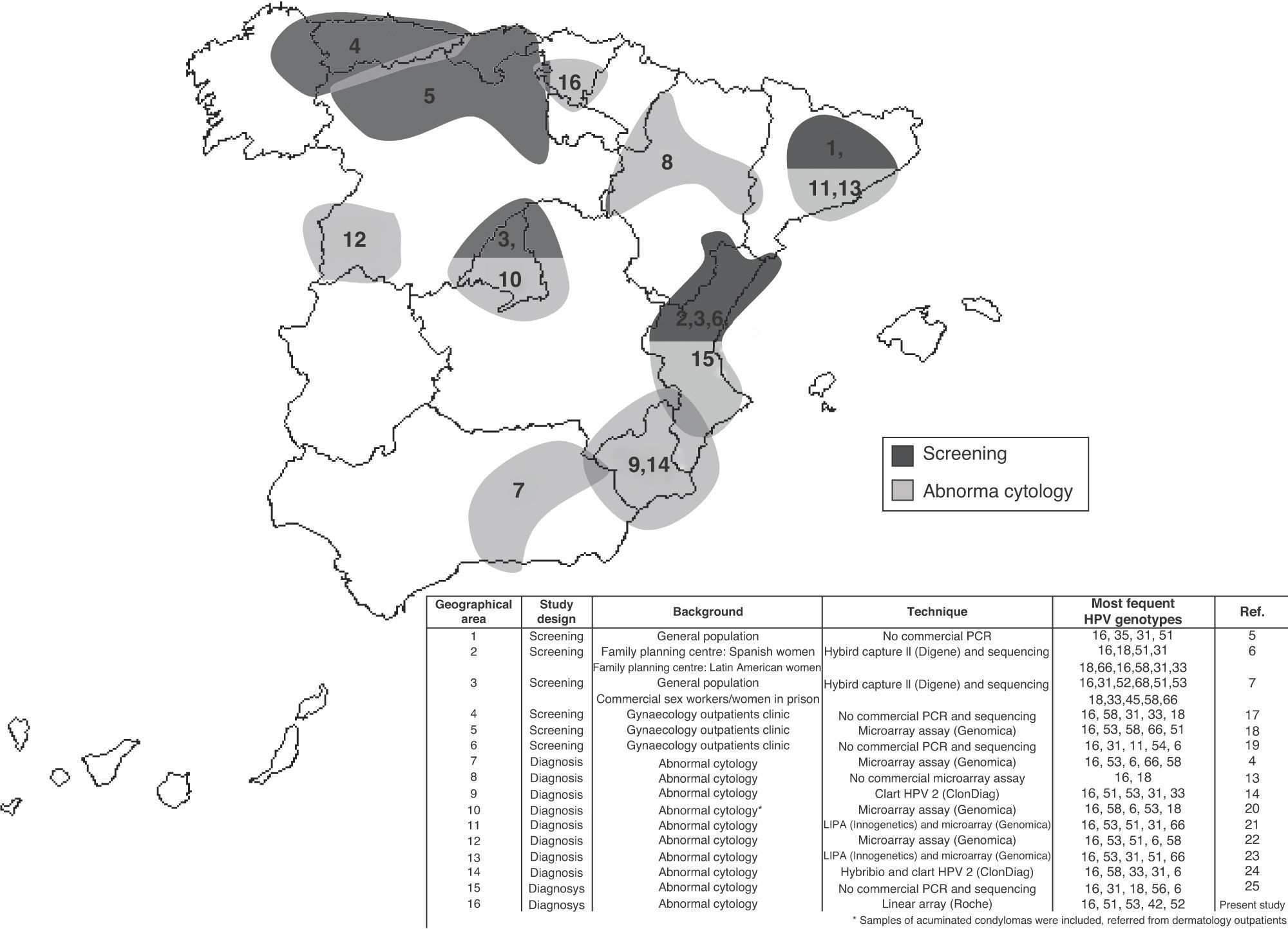
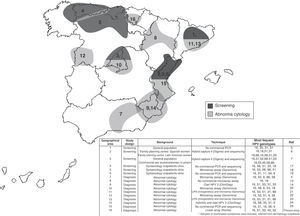
Fig. 1 shows the distribution of the more prevalent genotypes in Spain, including the results obtained in this study. Information on the study design, the background of the women included, the genotyping test used and the Spanish geographical area, are indicated in the figure.
DiscussionIn this study we have shown the following: first, 30 different HPV types were identified by Linear Array HPV genotyping in women with abnormal cytology, the HPV types 16, 51, 53, 42, 52, 39, 18 and 58 being the most common; second, the overall prevalence of HPV as well as the prevalence of HR HPV types increased with increasingly abnormal cytology; and third, the prevalence of HPV infection was higher in women aged 18–25 years.
An overall prevalence of 69.8% of HPV infection was found in cytologically abnormal cervical samples from women living in our region. These data are similar to those reported in other studies carried out in other Spanish regions and in other countries close to Spain, such as Italy.4,8,13,14 No HPV DNA was detected in 25% of HSIL samples; this may have been due to the presence of focal lesions not present in the material analyzed, or to possible undetectable genotypes or to the presence of low copy of HPV DNA.15 Most of the negative samples were associated with a cytological diagnosis of ASCUS or LSIL (93.7%), and may be explained by HPV clearance when epithelial changes have not yet fully regressed.16
Genotype 16 was the most prevalent, as found in other epidemiological studies on HPV, irrespective of the design, screening5–7,17–19 or detection in women with abnormal cytology.4,13,14,20–25 Worldwide, HPV 16 is the most common HPV type across the spectrum of HPV-related cervical lesions.2,10,26 The increase in the relative contribution of HPV 16 from 2% to 4% in women with normal cytology to 50–55% in invasive cervical cancer supports the notion of its biological advantage for transmission and transformation.1 In this study, genotype 16 reached 29.7% of the positive cases; this result is similar to that obtained in other studies that also evaluated abnormal cytologies, as can be observed in Fig. 1.4,13,14,20–25 In our study, the prevalence of the other genotypes is conditioned by the high proportion of patients with ASCUS or LSIL. Clifford et al.27 in a meta-analysis carried out with data from 55 studies of women with low-grade cervical lesions reported a similar prevalence to that obtained in our study, except for the genotype 31. This genotype was the second most prevalent in the Clifford study (15% approximately in Europe) and had a very low prevalence in our study (<3%). This may be related to migrant populations from high-risk countries with a higher prevalence of genotype 31, such as Central/South America and Eastern Europe.2,27 Therefore, this genotype is most prevalent in the Spanish regions with a high migrant population from these geographical areas, such as Madrid,7 Barcelona,21 Valencia25 and Murcia14,24 (5.6–8.2% from Central/South America and 0.03–3.8% from Eastern Europe28). However, in other regions, such as Salamanca22 and Alava, with lower migrant populations (<3% from Central/South America and <0.009% from Eastern Europe28), the prevalence of genotype 31 is lower.
In our study, 30 different HPV types were identified, and HPV types not included in commercial vaccines were detected more frequently than viruses included in the vaccines. Most of these viral genotypes had an oncogenic character; this is consistent with other studies performed in our country, both in screening studies5–7,17–19 and in women with abnormal cytology.4,13,14,20–25
Differences in the relative prevalence of HPV types might be related to the complex interplay between different HPV genotypes with host immunogenetic factors, or due to impairment in cellular immunity. Another explanation is that the use of different sets of primers in HPV PCR methods does not amplify all genotypes with exactly the same sensitivity.3
Although it is known that the infection by oncogenic types of HPV increases with the grade of the cytological lesion29 (50%, 80% and 85% for ASCUS, LSIL and HSIL, respectively), in the present work, neither the prevalence of HPV nor the prevalence of HR HPV types, increased significantly with the grade of the cytological lesion.
HPV types 18 and 45 were detected with lower frequency, similar to that in other countries of the south of Europe.2,10 In European women, HPV type 18 was detected in 0.7% of women with normal cytology and in 5.1% of women with low-grade lesions, whereas type 45 was found in 0.3% of women with normal cytology and in 2.5% of women with low-grade lesions; this compares with HPV 16 (2.3% and 19.4%, respectively).26,27 The under-representation of HPV 18 and HPV 45 detected in pre-neoplastic lesions and the early presentation of cases of invasive cervical cancer that are positive for genotypes 18, 45, and not only the genotype 16, may be indicative of a short time of progression to invasive cancer, with or without transition through the pre-invasive stages and lends support to a high early integration rate into the human genome.10 Interestingly, one or more of genotypes 16, 18 and 45 were detected in a quarter of the women with ASCUS and in a third of the women with LSIL. It is important to note that these genotypes are related to developing cancer at a much younger age (<50 years) than other types.
It is well known that the prevalence of HPV infection and the occurrence of multiple infection decrease with an increase in age.21,30 In our study, the prevalence of HPV co-infections peaked in women with LSILs. Among authors who have reported the percentage of coinfection associated with the cytological lesion, most described a peak in women with mild to moderate abnormalities: ASCUS31 or LSIL32, and no association between multiple infection and severity of the lesion30 was detected. However, whether the co-infection implies a higher risk of cancer is still unresolved.10,26
Persistent HPV increases the risk of cervical abnormalities and women with short-term persistent HPV 16 are at high risk for cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3),33 although the persistence alone is not sufficient for carcinogenicity of the cervix. Most incidents of infection in young women either clear or demonstrate CIN3 within five years. CIN3 lesions can develop very quickly (within 2–3 years) following HPV exposure.26 CIN3 lesions are initially very small and it takes years for them to grow enough for detection by cytology and then colposcopy.34 Following HPV infection, prognosis is significantly linked to the HPV type. The early presentation of cases of invasive cervical cancer that were positive for HPV 16, 18 or 45 suggests that women with ASCUS or LSIL who are positive for these genotypes in the Linear Array HPV genotyping should be offered an increase in surveillance, including the endocervical zone, which is the site of most adenocarcinomas, or more aggressive management in selected patients at risk of loss-to-follow-up.10,34
Our study has various limitations. First, this well-validated set of primers does not amplify all genotypes with exactly the same sensitivity, and such differences remain a source of variation in the detection of genotypes. However, the Linear Array HPV Genotyping Test used in the present study has been studied extensively; it is robust and has been standardized for routine microbiology.35 Second, we have studied a small sample of the HSIL cases and cervical cancer. Third, we could not evaluate the influence of the HPV vaccine. The HPV vaccine was introduced in Spain in 2008 and the target population is the 14-year-old female group. This means that the HPV genotype distribution is not yet affected by the vaccination program. Moreover, due to the low proportion of migrant women in our sample (<5%), we could not evaluate the influence of this population that could be responsible for a higher genotypes distribution variation. The low proportion of migrant women is justified by the low level of immigration in this Spanish geographical area.
Conflict of interestThe authors declare no conflicts of interest.