The aim of this study was to investigate the incidence and risk factors for the development of AIDS-defining cancers (ADCs); and to investigate the effect of making different assumptions on the definition of incident cases.
MethodsA multicentre cohort study was designed. Poisson regression was used to assess incidence and risk factors. To account for misclassification, incident cases were defined using lag-times of 0, 14 and 30 days after enrolment.
ResultsA total of 6393 HIV-positive subjects were included in the study. The incidences of ADCs changed as the lag periods were varied from 0 to 30 days. Different risk factors emerged as the definition of incident cases was changed. For a lag time of 0, the risk of Kaposi sarcoma [KS] and non-Hodgkin lymphoma [NHL] increased at CD4 counts <200/ml. HAART was associated with lower risk of NHL and KS. Men who had sex with men had a higher risk of KS. KS and NHL were not associated with viral load, gender, or hepatitis B or C. The results were similar for a lag-time of 14 and 30 days; however, hepatitis C was significantly associated with NHL.
ConclusionsThis analysis shows the importance of the definition of incident cases in cohort studies. Alternative definitions gave different incidence estimates, and may have implications for the analysis of risk factors.
El objetivo de este estudio es analizar la incidencia y factores de riesgo para el desarrollo de carcinomas definitorios de SIDA (CDS), e investigar el efecto de diferentes definiciones de casos incidentes.
MétodosSe diseñó un estudio de cohortes. Se analizaron incidencia y factores de riesgo mediante regresión de Poisson. Se realizó el análisis para tres definiciones de casos incidentes: retraso de 0, 14 y 30 días desde la inclusión en la cohorte.
ResultadosSe incluyeron 6393 sujetos con infección por VIH. La incidencia de CDS cambió según las definiciones de casos incidentes (retraso 0, 14 y 30 días). Diferentes factores se asociaron con el riesgo de CDS para diferentes definiciones de casos incidentes. Para el retraso 0, el riesgo de sarcoma de Kaposi (KS) y linfoma no Hodgkin (LNH) fue mayor para valores de CD4 <200/ml. La terapia HAART se asoció a un menor riesgo de LNH y SK. Los hombres que tienen sexo con hombres tuvieron mayor riesgo de SK. SK y LNH no se asociaron con carga viral, género, hepatitis B o C. Para los retrasos de 14 y 30 días, los resultados fueron similares; sin embargo, la hepatitis C se asoció de forma significativa con el LNH.
ConclusionesNuestro estudios muestra la importancia de la definición de casos incidentes en los estudios de cohortes. Diferentes definiciones pueden influir en la estimación de la incidencia de CDS y en el análisis de los factores de riesgo.
Patients with human immunodeficiency virus (HIV) infection have an increased risk of various cancers.1 The epidemiology of AIDS-defining cancers (ADCs; Kaposi sarcoma [KS], non-Hodgkin lymphoma [NHL] and invasive cervical cancer [CC]) has changed during recent years, as highly active antiretroviral therapy (HAART) has reduced the incidence and improved the prognosis of both KS and NHL.1–4 Meanwhile, the incidence of several non-AIDS-defining malignancies, such as anal carcinoma, lung cancer, or hepatocarcinoma, has increased among patients with HIV infection.1,5,6 Patients infected with HIV also have a higher risk of Hodgkin lymphoma compared to the general population5; although no increases have been found over recent years in the HAART era.6
The higher incidence of malignancies in HIV-infected patients has been explained by several possible causes, such as HIV-induced immunosuppression, chronic antigenic stimulation or the increased exposure to carcinogens, such as tobacco. Several oncogenic viruses are related to ADCs: the role of human herpes virus 8 and human papillomavirus infection in the pathogenesis of KS and CC, respectively, is widely accepted. Epstein–Barr virus and human herpes virus 8 could have a role in the pathogenesis of AIDS-related NHL through chronic antigen stimulation of B lymphocytes.7 Recently, an association has been found between NHL and hepatitis C virus (HCV) in the general population,8 although the role of HCV in the pathogenesis of AIDS-related NHL is still controversial.
The epidemiology of HIV infection has specific characteristics in Spain compared to other European countries9 which could influence the incidence and risk factors associated with ADCs in our population. Until the last decade, the main risk factor for transmission in Spain was the use of intravenous drugs, and there is a high proportion of patients infected with hepatitis C virus (HCV).10,11 However, studies assessing the epidemiology of ADCs at national level are lacking, since most are done in a single institution12,13 or using regional AIDS registries,14–16 providing limited information on incidence and risk factors.
To investigate the incidence of ADC in cohort studies, prevalent cases (i.e. patients experiencing symptoms of the event of interest before or at enrolment) need to be excluded from the analysis. However, patients presenting with an ADC during the first days of follow-up might not be true incident cases; a patient may have an ADC on enrolment, but could be diagnosed several days later after evaluating the results of radiological studies or biopsy material. The issue of defining prevalent and incident cases to account for diagnostic delay of ADCs (or other AIDS-defining events), and its impact on the estimates of incidence and the analysis of risk factors have not yet been addressed in the literature.
The aims of this study were to investigate the incidence and risk factors for the diagnosis of ADCs in two Spanish multicentre cohorts, and to investigate the effect of making different assumptions on the definition of incident cases.
Patients and methodsPatients and data collectionCoRIS and CoRIS-MD are two Spanish multicentre, clinical-based cohorts of HIV-seroprevalent subjects which have been described in previous publications.17,18 Briefly, CoRIS-MD is a cohort assembled in 2003–2004 that included all subjects aged over 18 years, with at least 6 months of follow-up, seen between January 1st, 1997 and June 30th, 2003 in 10 participating hospitals from 7 of the 17 Spanish Autonomous Regions, irrespective of their treatment status. CoRIS is a prospective cohort that included antiretroviral-naive subjects aged over 13 years seen in 29 hospitals (which included the 10 hospitals from CoRIS-MD plus 19 new hospitals) from 13 Autonomous Regions from January 2004. The administrative censoring date for these analyses was 31 December 2003 for CoRIS-MD and 31 October 2008 for CoRIS.
We included all naive patients from CoRIS and CoRIS-MD enrolled between 1 January 1997 and 1 July 2008. In order to minimise possible differences between the two cohorts, we included only patients who fulfilled the inclusion criteria for both: only patients over 18 years and at least with 6 months of follow-up were included. We collected data for the following variables: age at enrolment, sex, transmission categories (male homosexual contact, injecting drug users [IDUs], heterosexual contacts, other/unknown), calendar period of observation (1997–2000, 2001–2004, and 2005–2008), CD4 cell count at enrolment, viral load at enrolment, vital status, occurrence of ADCs (KS, NHL or CC), hepatitis B at enrolment (defined as positive HbsAg), hepatitis C at enrolment (defined as positive HCV antibodies), and changes of treatment over time. HAART was defined as antiretroviral drug regimens including two nucleoside reverse transcriptase inhibitors (NRTI) plus either a non-nucleoside reverse transcriptase inhibitor, a protease inhibitor, or three NRTIs. HAART was modelled as a time-dependent covariate with an intention-to-treat analysis (once started on HAART, patients were assumed to remain on it).
Statistical analysisWe used descriptive statistics to compare baseline characteristics between subjects with and without AIDS-defining cancers before or at enrolment. We used Fisher's exact test to compare proportions and Mann–Whitney U-test to compare continuous variables. All tests were two-sided, and a P<.05 was considered significant.
ADCs were diagnosed when the patient developed signs or symptoms of KS, NHL or CC, and the diagnosis was confirmed by the clinician taking care of the patient. Rates of ADCs were calculated as the number of new cases per 1000 person-years. Subjects were followed up from the date of enrolment until the date of first diagnosis of KS, NHL or CC, or the date of last visit, death, or censoring. All recurrences of ADCs were ignored. However, a previous episode of a different ADC from the one analysed did not prevent a patient from entering the risk set (for example, a patient with previous KS was not excluded for the analysis of NHL). All prevalent cases were excluded from the analysis. To account for possible misclassification of prevalent cases as incident cases, we used three alternative definitions of incident cases: lag 0 (incident cases defined as those diagnosed with ADC at any time after enrolment), lag 14 (incident cases defined as those diagnosed with ADC>14 days after enrolment), and lag 30 (incident cases defined as those diagnosed with ADC>30 days after enrolment). We performed all statistical analysis for every definition of incident cases. For each lag period, incidence rates and hazard ratios were calculated using the different number of person-years at risk from day 0, day 14 and day 30, respectively, as denominators.
We used multivariable Poisson regression to evaluate the relative rates for the development of ADCs. Robust methods were used to estimate confidence intervals (CI), assuming correlation between the subjects in each centre. Wald tests were used to calculate P values. All statistical analyses were performed in Stata version 9.0 (College Station, TX, USA).
EthicsEthics committee's approval was obtained from all participating hospitals. All subjects in CoRIS signed informed consent to participate in the cohort.
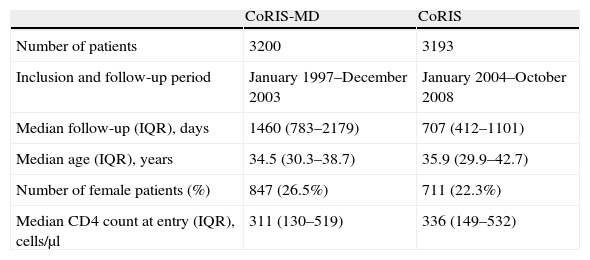
ResultsA total of 6393 naïve patients were included (3193 from CoRIS and 3200 from CoRIS-MD). The major characteristics and time of follow-up of each cohort are shown in Table 1.
Major demographic characteristics and time of follow-up for patients included from each cohort.
| CoRIS-MD | CoRIS | |
| Number of patients | 3200 | 3193 |
| Inclusion and follow-up period | January 1997–December 2003 | January 2004–October 2008 |
| Median follow-up (IQR), days | 1460 (783–2179) | 707 (412–1101) |
| Median age (IQR), years | 34.5 (30.3–38.7) | 35.9 (29.9–42.7) |
| Number of female patients (%) | 847 (26.5%) | 711 (22.3%) |
| Median CD4 count at entry (IQR), cells/μl | 311 (130–519) | 336 (149–532) |
IQR: interquartile range.
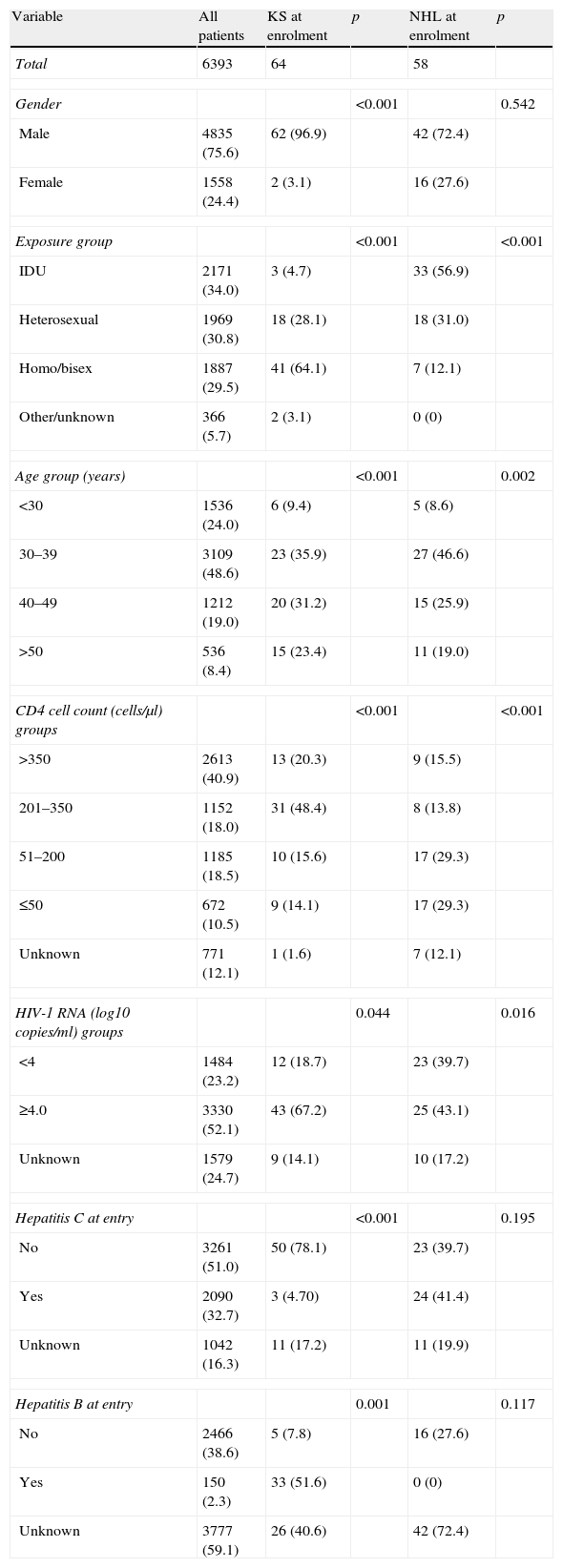
Sixty-four patients had KS, 58 had NHL and 3 had CC before or at enrolment. Demographic and clinical characteristics of all patients in the cohort and patients with KS and NHL before or at enrolment are shown in Table 2.
Demographic and clinical characteristics of all patients in the cohort, patients with Kaposi sarcoma at enrolment, and patients with non-Hodgkin lymphoma at enrolment.
| Variable | All patients | KS at enrolment | p | NHL at enrolment | p |
| Total | 6393 | 64 | 58 | ||
| Gender | <0.001 | 0.542 | |||
| Male | 4835 (75.6) | 62 (96.9) | 42 (72.4) | ||
| Female | 1558 (24.4) | 2 (3.1) | 16 (27.6) | ||
| Exposure group | <0.001 | <0.001 | |||
| IDU | 2171 (34.0) | 3 (4.7) | 33 (56.9) | ||
| Heterosexual | 1969 (30.8) | 18 (28.1) | 18 (31.0) | ||
| Homo/bisex | 1887 (29.5) | 41 (64.1) | 7 (12.1) | ||
| Other/unknown | 366 (5.7) | 2 (3.1) | 0 (0) | ||
| Age group (years) | <0.001 | 0.002 | |||
| <30 | 1536 (24.0) | 6 (9.4) | 5 (8.6) | ||
| 30–39 | 3109 (48.6) | 23 (35.9) | 27 (46.6) | ||
| 40–49 | 1212 (19.0) | 20 (31.2) | 15 (25.9) | ||
| >50 | 536 (8.4) | 15 (23.4) | 11 (19.0) | ||
| CD4 cell count (cells/μl) groups | <0.001 | <0.001 | |||
| >350 | 2613 (40.9) | 13 (20.3) | 9 (15.5) | ||
| 201–350 | 1152 (18.0) | 31 (48.4) | 8 (13.8) | ||
| 51–200 | 1185 (18.5) | 10 (15.6) | 17 (29.3) | ||
| ≤50 | 672 (10.5) | 9 (14.1) | 17 (29.3) | ||
| Unknown | 771 (12.1) | 1 (1.6) | 7 (12.1) | ||
| HIV-1 RNA (log10copies/ml) groups | 0.044 | 0.016 | |||
| <4 | 1484 (23.2) | 12 (18.7) | 23 (39.7) | ||
| ≥4.0 | 3330 (52.1) | 43 (67.2) | 25 (43.1) | ||
| Unknown | 1579 (24.7) | 9 (14.1) | 10 (17.2) | ||
| Hepatitis C at entry | <0.001 | 0.195 | |||
| No | 3261 (51.0) | 50 (78.1) | 23 (39.7) | ||
| Yes | 2090 (32.7) | 3 (4.70) | 24 (41.4) | ||
| Unknown | 1042 (16.3) | 11 (17.2) | 11 (19.9) | ||
| Hepatitis B at entry | 0.001 | 0.117 | |||
| No | 2466 (38.6) | 5 (7.8) | 16 (27.6) | ||
| Yes | 150 (2.3) | 33 (51.6) | 0 (0) | ||
| Unknown | 3777 (59.1) | 26 (40.6) | 42 (72.4) | ||
Figures indicate n (%) unless stated otherwise. KS: Kaposi sarcoma; NHL: non-Hodgkin lymphoma; IDU: injecting drug user.
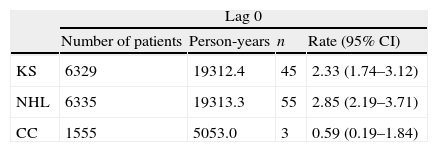
After excluding patients with KS, NHL or CC before or at enrolment, there were 45 patients who were diagnosed with KS, 55 patients with NHL, and 3 patients with CC during the follow-up period. We assessed the sensitivity of our results to the definition of incident cases by using lag periods of 0, 14 and 30 days. The number of incident cases and the incidence rates of ADC for each lag period are shown in Table 3. For lag 0, there was an annual incidence of 2.33 cases of KS, 2.85 cases of NHL and 0.59 cases of CC per 1000 population. The incidence of KS and NHL decreased slightly for increasing lag periods (Table 3). There were 16 cases of primary cerebral lymphoma for lag period 0, with an annual incidence of 0.82 (95% CI: 0.50–1.34) cases per 1000 population. For lag 14 and 30, there were 13 and 11 cases of primary cerebral lymphoma, respectively, giving an annual incidence of 0.67 (95% CI: 0.39–1.15) and 0.57 (95% CI: 0.31–1.02) cases per 1000 population.
Number of patients included, person-years at risk, and number of events and rates per 1000 person-years of KS, NHL and CC for each lag period.
| Lag 0 | ||||
| Number of patients | Person-years | n | Rate (95% CI) | |
| KS | 6329 | 19312.4 | 45 | 2.33 (1.74–3.12) |
| NHL | 6335 | 19313.3 | 55 | 2.85 (2.19–3.71) |
| CC | 1555 | 5053.0 | 3 | 0.59 (0.19–1.84) |
| Lag 14 | ||||
| Number of patients | Person-years | n | Rate (95% CI) | |
| KS | 6319 | 19312.2 | 35 | 1.81 (1.30–2.52) |
| NHL | 6327 | 19313.2 | 47 | 2.43 (1.83–3.24) |
| CC | 1555 | 5053.0 | 3 | 0.59 (0.19–1.84) |
| Lag 30 | ||||
| Number of patients | Person-years | n | Rate (95% CI) | |
| KS | 6315 | 19311.9 | 31 | 1.60 (1.13–2.28) |
| NHL | 6323 | 19312.9 | 43 | 2.23 (1.65–3.00) |
| CC | 1555 | 5053.0 | 3 | 0.59 (0.19–1.84) |
KS: Kaposi sarcoma; NHL: non-Hodgkin lymphoma; CC: invasive cervical cancer; n: number of events; CI: confidence interval.
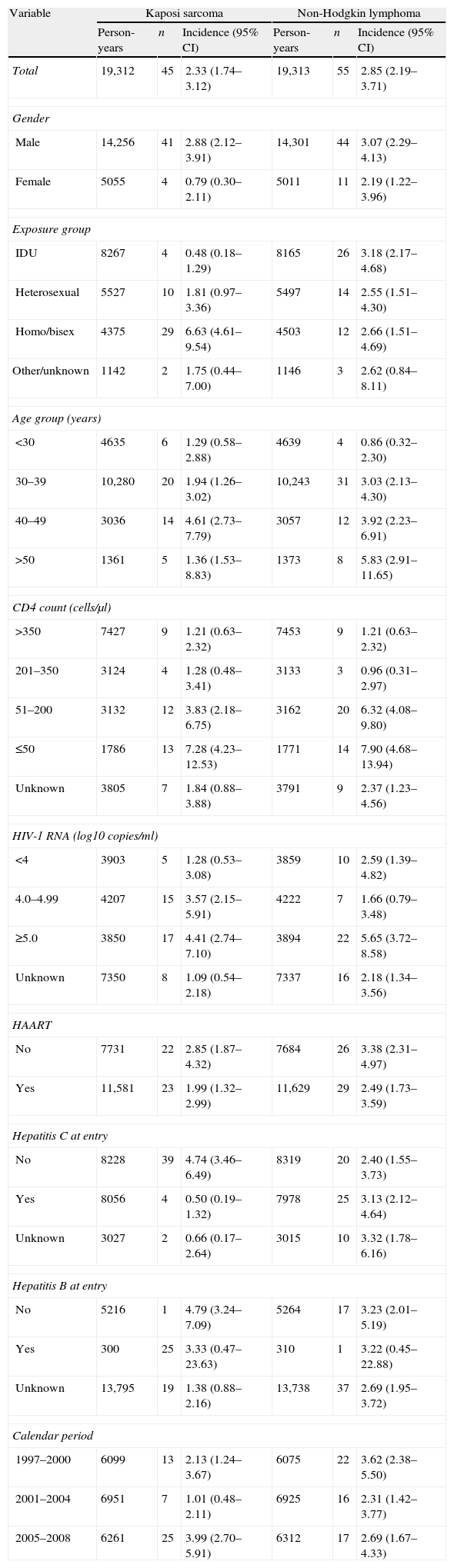
The incidence rates of KS and NHL divided by each risk factor category for lag period 0 days are shown in Tables 4–6 show the multivariable analyses of factors associated with KS and NHL for each lag period. Due to the low number of cases, we did not perform these analyses for CC or primary cerebral lymphoma. For the purpose of analysis of risk factors, the cases of primary cerebral lymphoma were included in the NHL group.
Incidence rates of Kaposi sarcoma and non-Hodgkin lymphoma (cases per 1000 person-years) for lag period 0 days.
| Variable | Kaposi sarcoma | Non-Hodgkin lymphoma | ||||
| Person-years | n | Incidence (95% CI) | Person-years | n | Incidence (95% CI) | |
| Total | 19,312 | 45 | 2.33 (1.74–3.12) | 19,313 | 55 | 2.85 (2.19–3.71) |
| Gender | ||||||
| Male | 14,256 | 41 | 2.88 (2.12–3.91) | 14,301 | 44 | 3.07 (2.29–4.13) |
| Female | 5055 | 4 | 0.79 (0.30–2.11) | 5011 | 11 | 2.19 (1.22–3.96) |
| Exposure group | ||||||
| IDU | 8267 | 4 | 0.48 (0.18–1.29) | 8165 | 26 | 3.18 (2.17–4.68) |
| Heterosexual | 5527 | 10 | 1.81 (0.97–3.36) | 5497 | 14 | 2.55 (1.51–4.30) |
| Homo/bisex | 4375 | 29 | 6.63 (4.61–9.54) | 4503 | 12 | 2.66 (1.51–4.69) |
| Other/unknown | 1142 | 2 | 1.75 (0.44–7.00) | 1146 | 3 | 2.62 (0.84–8.11) |
| Age group (years) | ||||||
| <30 | 4635 | 6 | 1.29 (0.58–2.88) | 4639 | 4 | 0.86 (0.32–2.30) |
| 30–39 | 10,280 | 20 | 1.94 (1.26–3.02) | 10,243 | 31 | 3.03 (2.13–4.30) |
| 40–49 | 3036 | 14 | 4.61 (2.73–7.79) | 3057 | 12 | 3.92 (2.23–6.91) |
| >50 | 1361 | 5 | 1.36 (1.53–8.83) | 1373 | 8 | 5.83 (2.91–11.65) |
| CD4 count (cells/μl) | ||||||
| >350 | 7427 | 9 | 1.21 (0.63–2.32) | 7453 | 9 | 1.21 (0.63–2.32) |
| 201–350 | 3124 | 4 | 1.28 (0.48–3.41) | 3133 | 3 | 0.96 (0.31–2.97) |
| 51–200 | 3132 | 12 | 3.83 (2.18–6.75) | 3162 | 20 | 6.32 (4.08–9.80) |
| ≤50 | 1786 | 13 | 7.28 (4.23–12.53) | 1771 | 14 | 7.90 (4.68–13.94) |
| Unknown | 3805 | 7 | 1.84 (0.88–3.88) | 3791 | 9 | 2.37 (1.23–4.56) |
| HIV-1 RNA (log10copies/ml) | ||||||
| <4 | 3903 | 5 | 1.28 (0.53–3.08) | 3859 | 10 | 2.59 (1.39–4.82) |
| 4.0–4.99 | 4207 | 15 | 3.57 (2.15–5.91) | 4222 | 7 | 1.66 (0.79–3.48) |
| ≥5.0 | 3850 | 17 | 4.41 (2.74–7.10) | 3894 | 22 | 5.65 (3.72–8.58) |
| Unknown | 7350 | 8 | 1.09 (0.54–2.18) | 7337 | 16 | 2.18 (1.34–3.56) |
| HAART | ||||||
| No | 7731 | 22 | 2.85 (1.87–4.32) | 7684 | 26 | 3.38 (2.31–4.97) |
| Yes | 11,581 | 23 | 1.99 (1.32–2.99) | 11,629 | 29 | 2.49 (1.73–3.59) |
| Hepatitis C at entry | ||||||
| No | 8228 | 39 | 4.74 (3.46–6.49) | 8319 | 20 | 2.40 (1.55–3.73) |
| Yes | 8056 | 4 | 0.50 (0.19–1.32) | 7978 | 25 | 3.13 (2.12–4.64) |
| Unknown | 3027 | 2 | 0.66 (0.17–2.64) | 3015 | 10 | 3.32 (1.78–6.16) |
| Hepatitis B at entry | ||||||
| No | 5216 | 1 | 4.79 (3.24–7.09) | 5264 | 17 | 3.23 (2.01–5.19) |
| Yes | 300 | 25 | 3.33 (0.47–23.63) | 310 | 1 | 3.22 (0.45–22.88) |
| Unknown | 13,795 | 19 | 1.38 (0.88–2.16) | 13,738 | 37 | 2.69 (1.95–3.72) |
| Calendar period | ||||||
| 1997–2000 | 6099 | 13 | 2.13 (1.24–3.67) | 6075 | 22 | 3.62 (2.38–5.50) |
| 2001–2004 | 6951 | 7 | 1.01 (0.48–2.11) | 6925 | 16 | 2.31 (1.42–3.77) |
| 2005–2008 | 6261 | 25 | 3.99 (2.70–5.91) | 6312 | 17 | 2.69 (1.67–4.33) |
CI: confidence interval; IDU: injecting drug user.
Multivariable analysis of factors associated with Kaposi sarcoma for each lag period.a
| Lag 0 | Lag 14 | Lag 30 | ||||
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| Gender | 0.291 | 0.547 | 0.537 | |||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 0.56 (0.19–1.64) | 0.73 (0.26–2.04) | 0.72 (0.26–2.02) | |||
| Exposure group | <0.001 | 0.033 | 0.224 | |||
| IDU | 1.00 | 1.00 | 1.00 | |||
| Heterosexual | 1.74 (0.50–6.06) | 1.51 (0.39–5.79) | 1.46 (0.33–6.38) | |||
| Homo/bisex | 5.03 (1.16–21.84) | 4.30 (0.87–21.13) | 3.15 (0.56–17.64) | |||
| Other/unknown | 1.47 (0.34–6.46) | 1.60 (0.35–7.24) | 1.46 (0.31–6.82) | |||
| Age group (years) | 0.031 | 0.051 | 0.209 | |||
| <30 | 1.00 | 1.00 | 1.00 | |||
| 30–39 | 1.57 (0.83–2.98) | 2.04 (0.79–5.23) | 1.94 (0.66–5.66) | |||
| 40–49 | 2.74 (1.37–5.50) | 3.56 (1.45–8.74) | 2.71 (1.05–7.01) | |||
| >50 | 1.49 (0.62–3.55) | 2.10 (0.62–7.09) | 2.17 (0.68–6.97) | |||
| CD4 count (cells/μl) | <0.001 | 0.001 | 0.023 | |||
| >350 | 1.00 | 1.00 | 1.00 | |||
| 201–350 | 1.79 (0.59–5.44) | 1.19 (0.37–3.81) | 0.67 (0.18–2.46) | |||
| 51–200 | 6.88 (1.29–36.61) | 3.89 (0.55–27.43) | 2.85 (0.45–18.04) | |||
| ≤50 | 13.17 (3.31–52.44) | 5.98 (1.27–28.25) | 2.72 (0.61–12.09) | |||
| Unknown | 6.44 (2.29–18.15) | 5.61 (2.01–15.65) | 3.82 (1.38–10.53) | |||
| HIV-1 RNA (log10copies/ml) | 0.050 | 0.111 | NE | |||
| <4 | 1.00 | 1.00 | 1.00 | |||
| ≥4.0 | 1.81 (0.85–3.85) | 2.29 (0.86–6.09) | NE | |||
| Unknown | 0.73 (0.20–2.68) | 1.25 (0.24–6.57) | NE | |||
| HAART | 0.083 | 0.391 | 0.656 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.26 (0.06–1.19) | 0.48 (0.09–2.58) | 0.69 (0.13–3.60) | |||
| Hepatitis C at entry | 0.253 | 0.322 | 0.022 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.23 (0.04–1.32) | 0.28 (0.05–1.53) | 0.25 (0.05–1.28) | |||
| Unknown | 0.29 (0.05–1.51) | 0.33 (0.06–1.88) | 0.16 (0.04–0.60) | |||
| Hepatitis B at entry | 0.233 | 0.886 | 0.975 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.56 (0.10–3.02) | 0.84 (0.16–4.47) | 1.18 (1.20–7.02) | |||
| Unknown | 0.39 (0.10–1.52) | 0.71 (0.15–3.22) | 0.92 (0.17–4.90) | |||
RR: rate ratio; CI: confidence interval; IDU: injecting drug user; NE: not evaluable.
Multivariable analysis of factors associated with non-Hodgkin lymphoma for each lag period.a
| Lag 0 | Lag 14 | Lag 30 | ||||
| RR (95% CI) | p | RR (95% CI) | p | RR (95% CI) | p | |
| Gender | 0.993 | 0.512 | 0.460 | |||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 1.00 (0.66–1.50) | 0.84 (0.50–1.41) | 0.83 (0.50–1.37) | |||
| Exposure group | 0.832 | 0.633 | 0.949 | |||
| IDU | 1.00 | 1.00 | 1.00 | |||
| Heterosexual | 0.84 (0.46–1.55) | 0.80 (0.37–1.73) | 0.81 (0.35–1.87) | |||
| Homo/bisex | 1.05 (0.49–2.24) | 1.28 (0.58–2.85) | 0.95 (0.35–2.61) | |||
| Other/unknown | 0.84 (0.36–1.92) | 0.66 (0.20–2.22) | 0.77 (0.20–2.91) | |||
| Age group (years) | 0.089 | 0.061 | 0.035 | |||
| <30 | 1.00 | 1.00 | 1.00 | |||
| 30–39 | 2.82 (1.00–7.96) | 2.48 (0.89–6.89) | 2.53 (0.90–7.14) | |||
| 40–49 | 3.24 (0.80–13.15) | 2.21 (0.51–9.52) | 1.44 (0.29–7.10) | |||
| >50 | 5.34 (1.38–21.00) | 5.25 (1.28–21.45) | 4.64 (1.09–19.99) | |||
| CD4 count (cells/μl) | <0.001 | <0.001 | <0.001 | |||
| >350 | 1.00 | 1.00 | 1.00 | |||
| 201–350 | 0.94 (0.32–2.74) | 0.80 (0.26–2.48) | 0.52 (0.18–1.51) | |||
| 51–200 | 7.72 (2.37–25.21) | 5.11 (1.44–18.14) | 4.36 (1.27–15.00) | |||
| ≤50 | 10.28 (3.30–32.07) | 4.96 (1.24–19.87) | 4.37 (1.02–18.68) | |||
| Unknown | 2.60 (0.70–9.66) | 2.55 (0.61–10.56) | 2.33 (0.57–9.50) | |||
| HIV-1 RNA (log10copies/ml) | 0.261 | 0.324 | 0.348 | |||
| <4 | 1.00 | 1.00 | 1.00 | |||
| ≥4.0 | 1.10 (0.54–2.23) | 1.21 (0.50–2.93) | 1.06 (0.42–2.64) | |||
| Unknown | 0.54 (0.21–1.39) | 0.54 (0.18–1.60) | 0.51 (0.17–1.47) | |||
| HAART | <0.001 | 0.002 | 0.010 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.29 (0.17–0.49) | 0.47 (0.29–0.75) | 0.53 (0.33–0.86) | |||
| Hepatitis C at entry | 0.265 | 0.003 | 0.004 | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.40 (0.60–3.36) | 2.06 (1.22–3.48) | 1.82 (1.20–2.77) | |||
| Unknown | 1.84 (0.87–3.87) | 2.87 (1.46–5.65) | 2.88 (1.44–5.79) | |||
| Hepatitis B at entry | 0.782 | 0.612 | NE | |||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 0.90 (0.10–7.87) | 1.23 (0.13–11.33) | NE | |||
| Unknown | 0.43 (0.04–4.68) | 0.36 (0.04–3.51) | 0.45 (0.04–5.09) | |||
RR: rate ratio; CI: confidence interval; IDU: injecting drug user; NE: not evaluable.
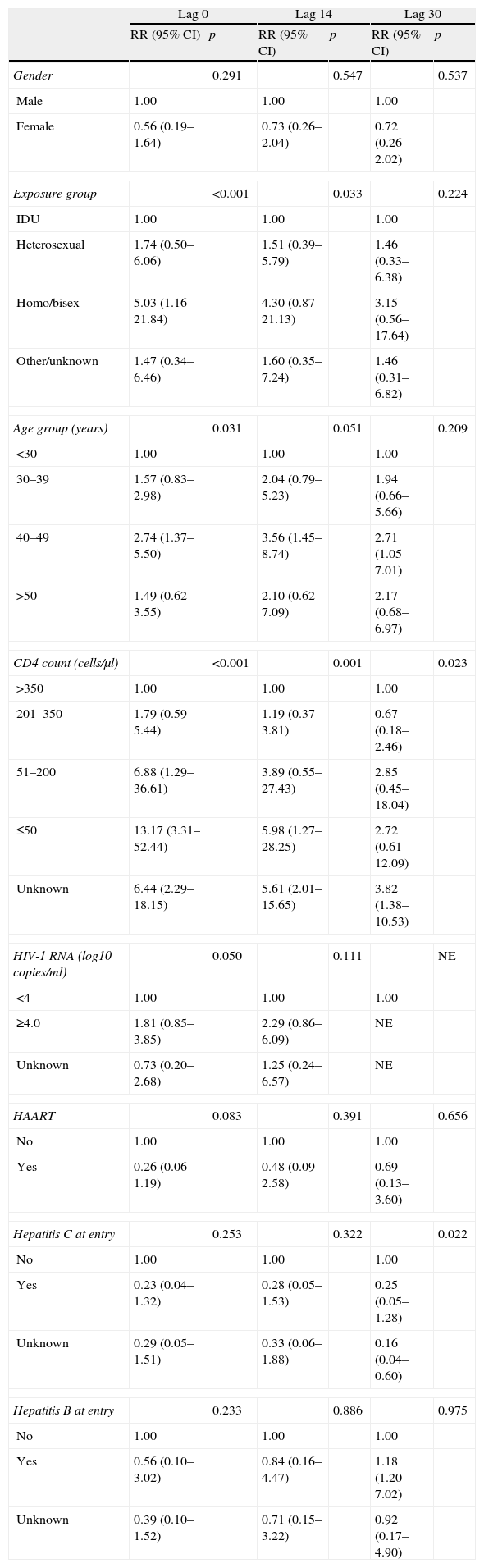
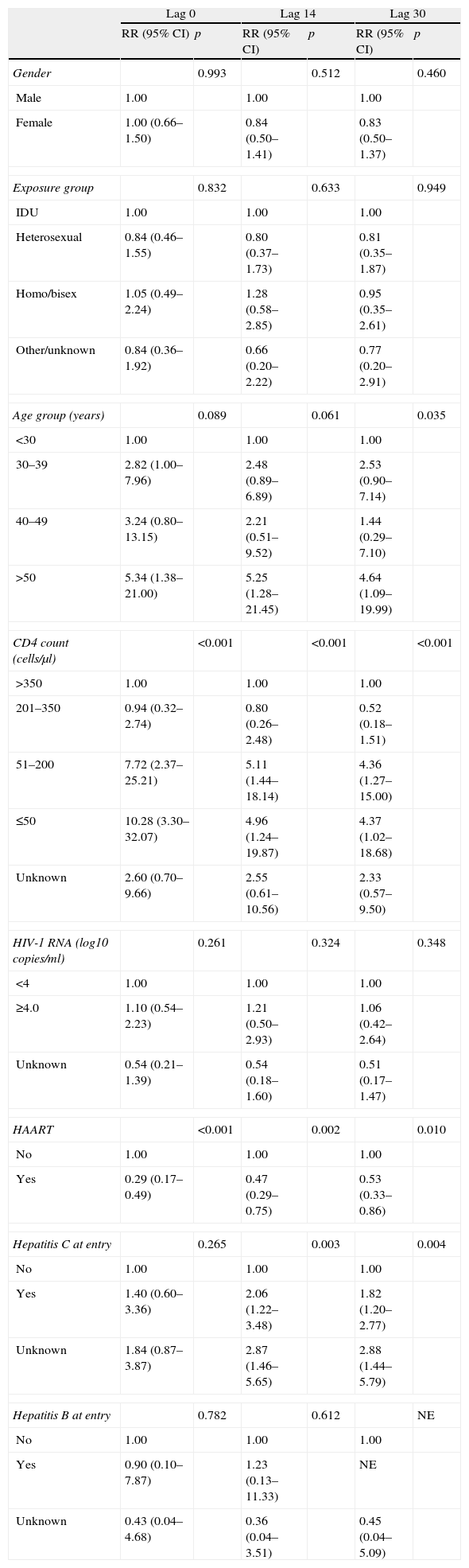
After adjusting for other risk factors, there was an increased risk of KS in homosexual patients and patients in the age group 40–49 years for lag 0. The risk of KS increased with CD4 counts <200cells/μl, and was highest for CD4 counts <50cells/μl. There was some evidence that HAART was associated with lower risk of KS, although this did not reach statistical significance (RR: 0.26, 95% CI: 0.06–1.19, P=.083). For lag 14, the effects of age group 40–49, CD4 counts <200cells/μl (and especially <50cells/μl), and male homosexual exposure groups on the increased risk of KS remained statistically significant. For lag 30, only the effect of CD4 cell count remained statistically significant, and a new association was found, as there was a lower risk of KS in patients with hepatitis C.
There was an increased risk of NHL for lag 0 in patients older than 50 years, and with CD4 counts <200cells/μl. HAART was strongly associated with a lower risk of NHL. These associations remained statistically significant for lags 14 and 30. There was no association between HCV or HBV and the risk of NHL for lag 0. However, HCV infection was associated with a higher risk of NHL for lags 14 and 30.
DiscussionOur study provides estimates of the incidence and risk factors for the diagnosis of ADCs in HIV-infected subjects from two large multicentre cohorts in Spain. To our knowledge, this is the first study that also investigates the effect of the definition of incident cases on the incidence and risk factor estimates. We found an annual incidence of 2.33 cases of KS, 2.85 cases of NHL and 0.59 cases of CC per 1000 population; there were slightly lower incidences of KS and NHL when using more strict criteria in the definition of incident cases, as expected. However, we also found that different risk factors emerged as we changed the definition of incident cases. This stresses the importance of accurately defining what is meant by incident and prevalent cases in HIV cohort studies.
The definition of incident cases is important because if a proportion of the (supposedly) incident cases are in reality prevalent cases, the incidence of ADC would be overestimated, and the relative risk estimates for different factors might be biased. None of the studies investigating the incidence of ADC, or other AIDS-defining events, have yet addressed this issue. We used lag periods of 14 and 30 days to assess the sensitivity of our results, as we assumed that it would be unlikely that a prevalent ADC would remain unnoticed after 30 days of follow-up. The choice of the cut-off points for the lag periods was done based on our experience on the usual delay in the results of diagnostic tests in most of the centres that participate in the cohort. As we used more strict criteria for the definition of incident cases, from lag 0 to lag 14 and 30, the incidence of KS and NHL decreased. This is explained because many events were diagnosed during the first 14 and 30 days (for example, almost one third of the KS cases were diagnosed during the first month after enrolment). Thus, the number of events decreased significantly with increasing lag periods, but the number of person-years as risk was minimally changed, as the contribution in time of the excluded patients for lags 14 and 30 was very small.
We have used clinical criteria for the definition of the diagnosis of ADC: an ADC was diagnosed when the patient developed signs or symptoms of KS, NHL or CC, and it was confirmed by the clinician in charge of the patient. It could be argued that when patients have an ADC diagnosed, the disease was already present in a subclinical stage for some time before, and the real date of the development of the cancer would be unknown. As it would be impossible to precisely know when each of the ADC could have started, we used a case definition based on clinical criteria (the development of signs and symptoms).
As found in other studies,1 NHL was the most frequent ADC, followed closely by KS. We found an increased risk of KS for patients aged 40–49 years, and of NHL for patients older than 50 years. These findings are similar to previous studies that found a higher risk of NHL with increasing age.3,19,20 The age groups with higher incidence of KS are more variable across different studies.13,19,21 The risk of KS was higher for homosexual patients, as is well described in the literature.1
The risk of developing KS or NHL was strongly associated with CD4 counts <200cells/μl at enrolment, and especially with CD4 counts <50cells/μl. This association was consistent across all lag periods. After adjusting for other risk factors, viral load was not associated with the risk of KS or NHL, suggesting that CD4 cell count is of greater importance for the occurrence of ADCs than viral load. Low CD4 cell counts have been consistently related to the risk of all ADCs,5,22 KS2,19 and NHL.19,20,23 However, while some studies find an association of ADCs with viral load,5,19,23 other studies do not find any association with viral load after adjusting for CD4 cell counts and other risk factors.2,24
The use of HAART was strongly associated with lower risk of NHL. There was some evidence of a lower risk of KS with the use of HAART for lag 0, although this did not reach statistical significance. Previous studies found a lower risk of KS and NHL in patients treated with HAART.6,19,20,23,25 Our findings are similar to those of Guiguet et al.19 in that there is a protective effect, even after adjusting for CD4 count and viral load, suggesting that the effect of HAART may be due to factors other than immune restoration: these authors suggested the antitumour activity of some antiretroviral drugs or a role of inflammation in the tumour process as possible explanations.19
In the general population, HCV infection has been recently reported to increase the risk of NHL,8 and the risk appears to be higher in populations with a high prevalence of HCV.8 However, this association has not been found amongst HIV-infected groups.19,26 The mechanism by which chronic HCV infection could increase the risk of NHL is not fully understood: it has been attributed to the chronic antigen stimulation of B cells by the HCV.8 In our cohort, which has a high HCV prevalence, we found that HCV increased the risk of NHL, but only for lags 14 and 30. The fact that the association of HCV and NHL is still not well established in the literature, and also that this effect is found in lags 14 and 30, but not lag 0, makes us interpret this finding with caution. However, this stresses the importance of the definition of prevalent cases in the analysis of risk factors for ADC. We also found a lower risk of KS in patients with HCV infection, although no firm conclusions can be made due to the low number of cases.
Our study has several strengths: it includes a large multicentre cohort which is representative of the population, as the Spanish health system is free of charge and equally accessible to all persons; the data have undergone rigorous quality control procedures; and we include a sensitivity analysis for the definition of incident cases. We have tried to minimise the limitations of the joint analysis of two different cohorts with slightly different inclusion criteria by restricting the analysis to the patients who fulfilled inclusion criteria for both cohorts. We are aware of the general limitations of cohort studies in HIV research.27 There is a possibility of competing risks in our analysis, as the patients dying from one ADC would not be able to experience a different ADC. However, we have not used methods to account for competing risks, since the deaths from ADCs were very few (data not shown) and it is highly unlikely that this would have biased our results.
Because our study was performed with a seroprevalent cohort, we were not able to adjust our results for the duration of HIV infection, as could be done with a seroconverter cohort. However, our results do not differ substantially from those found by recent studies with seroconverter cohorts: two studies found a higher risk of NHL28 and KS29 with lower current CD4 counts, and a lower risk of ADCs30 and KS29 has been found in patients receiving HAART.
Because patients were only included if they had a minimum follow-up of 6 months, some of the cases of ADC could have been missed, resulting in slightly lower incidence rate estimates. However, the number of cases that would have been excluded would be probably small, and it is unlikely that this would have biased our results. To further explore this, we performed an analysis with the patients from CoRIS, comparing our results with those obtained including patients with less than 6 months of follow-up (data not shown): the incidence rates and risk factors did not change substantially.
In conclusion, our main findings on incidence and association with risk factors for ADC in Spain are consistent with those found in other countries. As in other studies, we have found immunodeficiency and lack of antiretroviral therapy to be associated with a higher risk of KS and NHL. We have also explored a methodological problem on the definition of incident cases in epidemiological studies of ADC. The sensitivity analysis for different definitions of incident cases influenced our results: as the lag period increased from 0 to 14 and 30 days, so decreasing the number of incident cases, there was a slight decrease in the incidence of KS and NHL, and a decreased precision of the risk estimates for factors associated with their occurrence. Interestingly, associations such as that of HCV with NHL emerged with increasing lag periods. These findings call for more thorough considerations regarding the definition of incident cases.
Authors’ contributionsAll authors were involved in the setting up of the cohort and conceptualised the design. Julia del Amo, Inés Suárez-García and Inmaculada Jarrín asked the research question presented in this manuscript. All authors were involved in data collection. Inés Suárez-García was responsible for statistical analyses with support from Inmaculada Jarrín and Julia del Amo. Inés Suárez-García wrote the first draft of the paper, which was supervised by Julia del Amo. All authors were involved in interpretation of the data and commented on interim drafts. All authors have read and approved the final manuscript.
CoRIS-MD is integrated by:
Steering committee: Julia del Amo, Jesús Castilla, José Antonio Iribarren, Santiago Pérez-Hoyos, Maria Angeles Rodríguez Arenas and Santiago Moreno.
Analyses and writing committee: Santiago Moreno, Inmaculada Jarrín and Julia del Amo. Participating hospitals:Hospital Donostia, San Sebastián: J.A. Iribarren, J. Arrizabalaga, M.J. Aramburu, X. Camino, F. Rodríguez-Arrondo, M.A. von Wichmann; Hospital Ramón y Cajal, Madrid: Antonio Antela, José Luis Casado, Fernando Dronda, Ana Moreno, Santiago Moreno, M. Jesús Pérez Elías; Hospital Virgen del Rocío (I), Sevilla: Pompeyo Viciana Fernández, Luis Fernando López-Cortés, Mónica Trastoy González, Rosario Mata; Hospital Universitario de Granada: Alejandro Peña, Jorge Parra, Leopoldo Muñoz; Hospital Universitario de Canarias, Tenerife: Juan Luis Gómez Sirvent, M.ª Remedios Alemán Valls, M.ª del Mar Alonso Socas, Carlos Hernández Calzadilla; Hospital Universitari Joan XXIII, Tarragona. Universitat Rovira i Virgili: Maria Saumoy, Cristóbal Richart, Francesc Vidal; Hospital La Fe, Valencia: Cristina Falcó Couchoud, Marino Blanes Juliá, José Lacruz Rodrigo, Vicente Navarro Ibáñez, Miguel Salavert Lletí, José López Aldeguer; Hospital General de Elche, Alicante: Félix Gutiérrez, Sergio Padilla, Enrique Bernal, Mar Masiá; Hospital General de La Rioja, Logroño: José Antonio Oteo, José Ramón Blanco, Valvanera Ibarra; Hospital Virgen del Rocío (II), Sevilla: Manuel Leal Noval.
CoRIS is integrated by:
Steering committee: Juan Berenguer, Julia del Amo, Federico García, Félix Gutiérrez, Pablo Labarga, Santiago Moreno y María Ángeles Muñoz.
Analyses and writing committee: Ana María Caro-Murillo, Paz Sobrino Vegas, Santiago Pérez-Cachafeiro, Victoria Hernando Sebastián, Belén Alejos Ferreras, Débora Álvarez, Mónica Trastoy.
BioBank: M.ª Ángeles Muñoz-Fernández, Isabel García-Merino, Coral Gómez Rico, Jorge Gallego de la Fuente y Almudena García Torre.
Participating hospitals:Hospital General Universitario de Alicante (Alicante): Joaquín Portilla Sogorb, Esperanza Merino de Lucas, Sergio Reus Bañuls, Vicente Boix Martínez, Livia Giner Oncina, Carmen Gadea Pastor, Irene Portilla Tamarit, Patricia Arcaina Toledo; Hospital Universitario de Canarias (Santa Cruz de Tenerife): Juan Luis Gómez Sirvent, Patricia Rodríguez Fortúnez, María Remedios Alemán Valls, María del Mar Alonso Socas, Ana María López Lirola, María Inmaculada Hernández Hernández, Felicitas Díaz-Flores; Hospital Carlos III (Madrid): Vicente Soriano, Pablo Labarga, Pablo Barreiro, Carol Castañares, Pablo Rivas, Andrés Ruiz, Francisco Blanco, Pilar García, Mercedes de Diego; Hospital Universitario Central de Asturias (Oviedo): Victor Asensi, Eulalia Valle, José Antonio Cartón; Hospital Clinic (Barcelona): José M. Miró, María López-Dieguez, Christian Manzardo, Laura Zamora, Iñaki Pérez, M.ª Teresa García, Carmen Ligero, José Luis Blanco, Felipe García-Alcaide, Esteban Martínez, Josep Mallolas, José M. Gatell; Hospital Doce de Octubre (Madrid): Rafael Rubio, Federico Pulido, Silvana Fiorante, Jara Llenas, Violeta Rodríguez, Mariano Matarranz; Hospital Donostia (San Sebastián): José Antonio Iribarren, Julio Arrizabalaga, María José Aramburu, Xabier Camino, Francisco Rodríguez-Arrondo, Miguel Ángel von Wichmann, Lidia Pascual Tomé, Miguel Ángel Goenaga, M.ª Jesús Bustinduy, Harkaitz Azkune Galparsoro; Hospital General Universitario de Elche (Elche): Félix Gutiérrez, Mar Masiá, José Manuel Ramos, Sergio Padilla, Andrés Navarro, Fernando Montolio, Yolanda Peral, Catalina Robledano García; Hospital Germans Trías i Pujol (Badalona): Bonaventura Clotet, Cristina Tural, Lidia Ruiz, Cristina Miranda, Roberto Muga, Jordi Tor, Arantza Sanvisens; Hospital Gregorio Marañón (Madrid): Juan Berenguer, Juan Carlos López Bernaldo de Quirós, Pilar Miralles, Jaime Cosín Ochaíta, Matilde Sánchez Conde, Isabel Gutiérrez Cuellar, Margarita Ramírez Schacke, Belén Padilla Ortega, Paloma Gijón Vidaurreta; Hospital Universitari de Tarragona Joan XXIII, IISPV, Universitat Rovira i Virgili (Tarragona): Francesc Vidal, Joaquín Peraire, Consuelo Viladés, Sergio Veloso, Montserrat Vargas, Miguel López-Dupla, Montserrat Olona, Alba Aguilar, Joan Joseph Sirvent, Antoni Soriano, Rami AA. Qaneta; Hospital Universitario La Fe (Valencia): José López Aldeguer, Marino Blanes Juliá, José Lacruz Rodrigo, Miguel Salavert, Marta Montero, Eva Calabuig, Sandra Cuéllar; Hospital Universitário La Paz (Madrid): Juan González García, Ignacio Bernardino de la Serna, José María Peña Sánchez de Rivera, Marta Mora Rillo, José Ramón Arribas López, María Luisa Montes Ramírez, José Francisco Pascual Pareja, Blanca Arribas, Juan Miguel Castro, Fco Javier Zamora Vargas, Ignacio Pérez Valero; Hospital de la Princesa (Madrid): Ignacio de los Santos, Jesús Sanz Sanz, Johana Rodríguez, Ana Salas Aparicio, Cristina Sarriá Cepeda; Hospital San Pedro-CIBIR (Logroño): José Antonio Oteo, José Ramón Blanco, Valvanera Ibarra, Luis Metola, Mercedes Sanz, Laura Pérez-Martínez; Hospital San Pedro II (Logroño): Javier Pinilla Moraza; Hospital Universitario Mutua de Terrassa (Terrassa): David Dalmau, Angels Jaén Manzanera, Mireia Cairó Llobell, Daniel Irigoyen Puig, Laura Ibáñez, Queralt Jordano Montañez, Mariona Xercavins Valls, Javier Martinez-Lacasa, Pablo Velli, Roser Font;Hospital de Navarra (Pamplona): Julio Sola Boneta, Javier Uriz, Jesús Castiello, Jesús Reparaz, María Jesús Arraiza, Carmen Irigoyen, David Mozas; Hospital Parc Taulí (Sabadell): Ferrán Segura, María José Amengual, Eva Penelo, Gemma Navarro, Montserrat Sala, Manuel Cervantes, Valentín Pineda; Hospital Ramón y Cajal (Madrid): Santiago Moreno, José Luis Casado, Fernando Dronda, Ana Moreno, María Jesús Pérez Elías, Dolores López, Carolina Gutiérrez, Beatriz Hernández, María Pumares, Paloma Martí; Hospital Reina Sofía (Murcia): Alfredo Cano Sánchez, Enrique Bernal Morell, Ángeles Muñoz Pérez; Hospital San Cecilio (Granada): Federico García García, José Hernández Quero, Alejandro Peña Monje, Leopoldo Muñoz Medina, Jorge Parra Ruiz; Centro Sanitario Sandoval (Madrid): Jorge Del Romero Guerrero, Carmen Rodríguez Martín, Teresa Puerta López, Juan Carlos Carrió Montiel; Hospital Universitario Santiago de Compostela (Santiago de Compostela): Antonio Antela, Arturo Prieto, Elena Losada; Hospital Son Dureta (Palma de Mallorca): Melchor Riera, Javier Murillas, Maria Peñaranda, Maria Leyes, M.ª Angels Ribas, Antoni Campins, Concepcion Villalonga; Hospital Universitario de Valme (Sevilla): Juan Antonio Pineda, Eva Recio Sánchez, Fernando Lozano de León, Juan Macías, José del Valle, Jesús Gómez-Mateos, Rosario Mata; Hospital Virgen de la Victoria (Málaga): Jesús Santos González, Manuel Márquez Solero, Isabel Viciana Ramos, Rosario Palacios Muñoz; Hospital Universitario Virgen del Rocío (Sevilla): Pompeyo Viciana, Manuel Leal, Luis Fernando López-Cortés, Mónica Trastoy.
FundingThis study has been financed by ISCIII-RETICRD06/006 (Carlos III Health Institute (ISCIII) National Biobank Network (RETIC)).
Conflict of interestThe authors declare that they have no conflict of interest.