The last decade has witnessed significant advances in mycobacterial genomics and cellular research which have resulted in the development of two new blood tests, the enzyme-linked immunospot assay (ELISpot) (TSPOT.TB, Oxford Immunotec, Oxford, UK) and the enzyme-linked immunosorbent assay (ELISA) (QuantiFERON-TB Gold In-Tube, Cellestis, Carnegie, Australia). These tests, which are collectively known as interferon gamma release assays (IGRAs), detect latent tuberculosis infection (LTBI) by measuring interferon (IFN)-γ release in response to antigens present in Mycobacterium tuberculosis, but not bacille Calmette-Guerin (BCG) vaccine and most nontuberculous mycobacteria. This is done through enumeration of IFN-γ-secreting T cells (ELISpot) or by measurement of IFN-γ concentration (ELISA). The evidence base for these tests has expanded rapidly and now demonstrates that IGRAs are more specific than the tuberculin skin test (TST) as they are not confounded by previous BCG vaccination. In addition, with active tuberculosis (TB) as a surrogate for LTBI, it appears that the ELISA has a similar sensitivity to the TST, whereas the ELISpot is more sensitive. Using degree of exposure to TB as a surrogate for LTBI, both assays correlate at least as well with TB exposure as the TST. Recent longitudinal data have now demonstrated the prognostic power of positive IGRA results in recent contacts for the subsequent progression to active TB. Deployment of IGRAs, driven by new guidelines internationally, will impact on clinical practice in several ways. Their high specificity means that BCG-vaccinated individuals with a false-positive TST will not receive unnecessary preventive treatment, whereas improved sensitivity in individuals with weakened cellular immunity at highest risk of progressing to active TB (for example HIV-positive individuals) enables more reliable targeted testing and treatment of these vulnerable groups. The role of IGRAs in active TB is less clear but they may be useful as adjunctive tests in the diagnostic work-up of an individual with suspected TB. Finally, recent developments and future directions in IGRA development are reviewed.
La última década ha asistido a significativos avances de la genómica micobacteriana y de la investigación celular que han desembocado en el desarrollo de 2 nuevos estudios sanguíneos, la determinación inmunoenzimática de puntos (ELISpot) (TSPOT.TB, Oxford Immunotec, Oxford, Reino Unido) y el análisis inmunoenzimático por adsorción (ELISA) (QuantiFERON-TB Gold In-tube; Cellestis; Carnegie, Australia). Estas pruebas, que se conocen colectivamente como determinaciones de la liberación de interferón gamma (IGRA), detectan la infección tuberculosa latente (LTBI) midiendo la liberación del interferón (IFN)-γ como respuesta a los antígenos presentes en Mycobacterium tuberculosis, pero no en la vacuna con bacilo de Calmette y Guerin (BCG) ni en la mayoría de las micobacterias no tuberculosas. Esto se consigue mediante la identificación de las células T secretoras de IFN-γ (ELISpot) o la medición de la concentración de IFN-γ (ELISA). La base de evidencias para estas pruebas ha aumentado con rapidez y en la actualidad demuestra que las IGRA son más específicas que la prueba cutánea con tuberculina (TST), porque no sufren confusión por vacunación previa con BCG. Además, con la tuberculosis (TB) activa como sustituto de la LTBI, parece que ELISA tiene una sensibilidad similar a la TST, mientras que ELISpot es más sensible. Si tenemos en cuenta el grado de exposición a la TB como sustituto de la LTBI, ambas determinaciones muestran una correlación con la TB al menos tan buena como la TST. Recientes datos longitudinales han demostrado el poder pronóstico de unos resultados positivos de IGRA en los contactos recientes respecto a la ulterior progresión a TB activa. La generalización de las IGRA, impulsada en el plano internacional por las nuevas pautas, incidirá en la práctica clínica de distintas maneras. Su gran especificidad consigue que los individuos vacunados con BCG y con una TST falsamente positiva no reciban un tratamiento preventivo innecesario, mientras que la mayor sensibilidad en los individuos con inmunidad celular debilitada, en el máximo riesgo de progresar a una TB activa (p. ej., los individuos positivos al VIH) permite un estudio y tratamiento dirigido y más fiable en estos grupos vulnerables. El papel de las IGRA en la TB activa es menos evidente, pero puede ser una útil prueba ayudante en el estudio diagnóstico de un individuo con sospecha de padecer una TB. Por último, revisan los recientes desarrollos y las futuras direcciones del desarrollo de las IGRA.
Although the World Health Organisation (WHO) declared tuberculosis (TB) to be a global health emergency over 15 years ago,1 TB remains as one of the leading infectious causes of morbidity in the world, accounting for 8 to 10 million cases per year.2 Despite this expanding threat to global public health, our tools for the diagnosis and prevention of TB are over one hundred years old, and they are inadequate to control the epidemic.3 However, significant advances have recently been made in the diagnosis of latent TB infection (LTBI) which promise to substantially improve TB control.4
Latent TB infectionThe epidemiology of TB is distinct in that the reservoir of latently infected individuals, estimated to be a third of the world's population, is much larger than the number of active TB cases that occur.5 As a result it is becoming increasingly more evident, particularly in non-endemic countries where latently infected migrants and recently-infected contacts give rise to the bulk of the TB case-load, that effective identification and treatment of LTBI has the potential to reverse the increasing TB burden.6,7
The natural history of TB is complex and remains poorly understood. After establishing initial infection, tubercle bacilli may cause disease immediately, or they may lie dormant for many decades; evidence suggests only 5% to 10% of infected immunocompetent people go on to develop active disease over their lifetime.8 Diagnosis and treatment of LTBI is based on “targeted testing” to selectively identify persons at highest risk of progression from LTBI to active disease. This includes recently-infected individuals and all those with suppressed or immature immune systems, regardless of when they acquired infection.9
Diagnosis of Latent TB infectionDiagnosis of LTBI has hitherto been defined as a positive tuberculin skin test (TST) in an otherwise asymptomatic person exposed to tuberculosis with no clinical or radiological evidence of active disease. The TST is based on the detection of a cutaneous delayed-typed hypersensitivity response to purified protein derivative, a poorly defined mixture of more than two hundred Mycobacterium tuberculosis proteins. However, although the TST is cheap and widely utilised, it has several drawbacks. Antigenic cross-reactivity of purified protein derivative reduces specificity in individuals previously vaccinated with BCG and/or exposed to nontuberculous mycobacteria, resulting in false-positive results.10 In addition, the TST is acknowledged to have poor sensitivity in individuals with compromised immune systems (eg, patients with HIV infection or iatrogenic immunosuppression, and children), resulting in false-negative results.11 Additional difficulties are logistic and include the need for the test to be performed by a trained health care professional and the requirement for a return visit to have the result read.
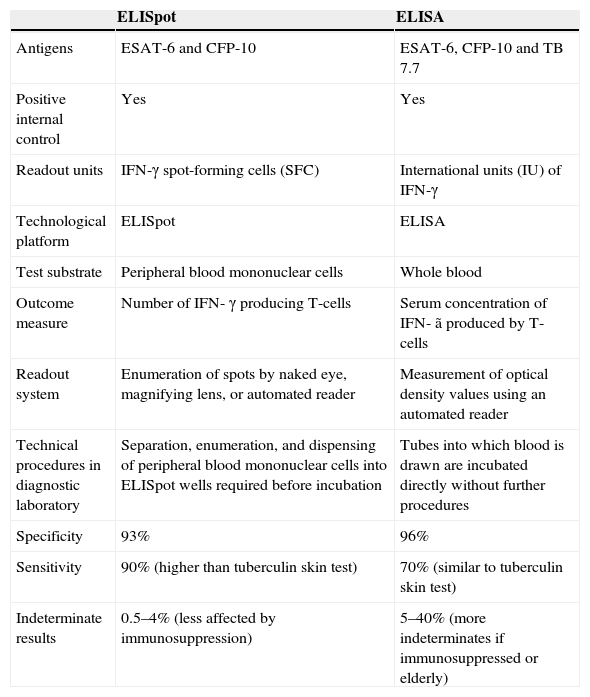
T-cell interferon-gamma release assaysT-cell interferon-gamma release assays (IGRAs) have been developed as an alternative immunodiagnostic approach to the TST for detecting M. tuberculosis infection.9,12,13 Their development has stemmed from advances in mycobacterial genomics which identified a genomic segment (Region of Difference 1) that is deleted from all strains of BCG14 and the majority of environmental mycobacteria (except M. Kansasi, M. szulgai, M. marinum, M. flavescens, and M. gastrii).15 Two antigens encoded by this segment, early secretory antigen target-6 (ESAT-6) and culture filtrate protein 10 (CFP-10), are strong targets of Th1 T-cells in M. tuberculosis infection; their ability to elicit strong, specific, T-cell responses reduces the frequency of false-positive TST results in individuals who have previously received BCG vaccination.3,16,17 This important property has been harnessed to form the basis of the two ex vivo IGRAs that are currently available: the ELISpot, which directly counts the number of IFN-γ secreting T cells, and the whole-blood ELISA, which measures the concentration of IFN-γ secretion. Both assays have been commercially licensed with the ELISpot assay available as the T-SPOT™-TB (Oxford Immunotec, Abingdon, UK) and the ELISA as the QuantiFERON™-TB Gold In-Tube (Cellestis, Carnegie, Australia). The main similarities and differences between the two assays are summarized in Table 1.
Similarities and differences between the ELISpot (TSPOT.TB) and ELISA (QuantiFERON-TB Gold In-tube)
| ELISpot | ELISA | |
| Antigens | ESAT-6 and CFP-10 | ESAT-6, CFP-10 and TB 7.7 |
| Positive internal control | Yes | Yes |
| Readout units | IFN-γ spot-forming cells (SFC) | International units (IU) of IFN-γ |
| Technological platform | ELISpot | ELISA |
| Test substrate | Peripheral blood mononuclear cells | Whole blood |
| Outcome measure | Number of IFN- γ producing T-cells | Serum concentration of IFN- ã produced by T-cells |
| Readout system | Enumeration of spots by naked eye, magnifying lens, or automated reader | Measurement of optical density values using an automated reader |
| Technical procedures in diagnostic laboratory | Separation, enumeration, and dispensing of peripheral blood mononuclear cells into ELISpot wells required before incubation | Tubes into which blood is drawn are incubated directly without further procedures |
| Specificity | 93% | 96% |
| Sensitivity | 90% (higher than tuberculin skin test) | 70% (similar to tuberculin skin test) |
| Indeterminate results | 0.5–4% (less affected by immunosuppression) | 5–40% (more indeterminates if immunosuppressed or elderly) |
Significant challenges exist in directly assessing whether IGRAs are superior to the TST in diagnosing LTBI as there is no available reference standard test. Studies attempting to quantify the test performance of IGRAs have therefore used surrogate markers for LTBI, including: (1) the level of contact with infectious cases, (2) active TB disease as a surrogate for LTBI and, to assess specificity, (3) IGRA-negative status in healthy individuals at low-risk of TB infection in low-prevalence settings.
Performance of interferon-gamma release assays in immunocompetent individualsCorrelation of interferon-gamma release assay results with TB exposureBecause airborne transmission and the subsequent risk of acquiring M. tuberculosis is primarily determined by the frequency, duration and proximity of contact with an infectious source case,18–21 it follows that if a new test is more sensitive and specific than the TST, it should correlate more closely with the level of exposure to M. tuberculosis and be independent of BCG status.22
A number of studies have utilised this principle to compare the diagnostic accuracy of IGRAs and the TST in outbreak and contact-tracing investigations, particularly amongst children.22–27 While the evidence base is more substantial for the ELISpot than the ELISA, the general consensus that can be drawn from these studies is that both IGRAs correlate either as well as, or better than, the TST with levels of exposure to tuberculosis whilst also being independent of BCG status. This was most clearly demonstrated in a large school outbreak investigation comprising 535 students, in which the ELISpot correlated significantly more closely with M. tuberculosis exposure than the TST based on proximity and duration of exposure to the index case.23 Other work from a variety of low-, intermediate-, and high-prevalence settings has also confirmed that the ELISpot significantly correlates with TB exposure.28–35 In a Turkish community-based study of household contacts, positive ELISpot and TST results significantly correlated with the index patient being a parent and the number of cases of smear-positive pulmonary tuberculosis per household.34 Most recently, in a study of 243 children in South Africa, positive ELISpot and TST results in children were found to be significantly associated with the degree of exposure to smear-positive index cases.33
Studies undertaken to explore the performance of the ELISA in children have shown that the ELISA significantly correlates with exposure to TB.28,36–40 In a recent study from New York, Lighter and colleagues found that with increasing gradients of exposure to M. tuberculosis, the proportion of children with positive ELISA results also increased.37 A Nigerian case-control study found that there was a dose-response relationship: children who had been in contact with the most heavily smear-positive index cases, rather than smear-negative adults, were more likely to be TST- and ELISA-positive.39 Similar results have been found in recent work from Korea and Cambodia.36,38 In contrast, a study from a South African township of children at high risk of LTBI did not find any significant relationship between levels of exposure and ELISA positivity.41
A further area of ongoing uncertainty that emerges from these studies is the level of concordance between the IGRAs and TST. In general, agreement between IGRAs and the TST is lower in BCG-vaccinated populations, as would be expected from the fact that the TST, but not IGRA, is confounded by BCG vaccination. Studies exploring levels of agreement have, on the whole, found moderate to high levels of agreement between the ELISpot assay and the TST.23,28,30,31,37 For example, in a UK study of contacts in school-based outbreak, the ELISpot and TST had high levels of agreement (κ=0.72)23 which was similar to the results from a Gambian study of child contacts (κ=0.62).31 In more recent data, levels of concordance have been moderate; Nicol and colleagues found a concordance of κ=0.55 between ELISpot and TST in South African children,33 whilst Australian investigators in a recent contact study also reported a similarly moderate level of concordance (κ=0.51) between the ELISpot and TST.28
Conversely, levels of agreement between the ELISA and the TST have been more variable, with kappa values ranging from 0.19 to 0.87.27,28,30,36–38,42,43 Whereas studies of hospitalised children in India (κ=0.73)42 and childhood contacts in Cambodia (κ=0.63)38 found a high levels of agreement between the ELISA and TST when used in the diagnosis of LTBI, other studies have reported considerably lower levels of concordance. In a South Korean case-control study the level of agreement between the ELISA and TST was low (κ=0.19).36 Similarly, in an Australian study there was poor agreement between the ELISA and TST (κ=0.3)40; in the 21 unvaccinated children who had a positive TST, only 4 were ELISA-positive. These findings appear to have been confirmed in a Spanish study in children who were not BCG vaccinated, where the ELISA was negative for 53.3% of children with a positive TST.30 These data suggest that the ELISA may have a lower sensitivity than the TST in diagnosing LTBI in children.
However, it should be borne in mind that these studies are cross-sectional; data from longitudinal studies will clarify the significance of discordant TST/IGRA results.
Active TB as a surrogate for latent tuberculosis infectionIn the absence of a reference standard test for LTBI, an alternative surrogate measure of diagnostic sensitivity of the IGRAs is to evaluate their performance in individuals with active TB disease who, by definition, are infected with TB. A recent systematic review using this methodology found that the ELISpot had a pooled sensitivity of 90% (range 83–100%),30,35,44–54 which was significantly higher than the pooled sensitivities of the second generation ELISA (78%, range 55–88%)13,43,45,48,49,52,53,55–63 and the latest generation ELISA (QuantiFERON-Gold in-tube, pooled 70%, range 64–93%).30,35,64–67
Performance of interferon-gamma release assays in immunocompromised individualsCorrectly identifying and treating LTBI in high-risk individuals with impaired cell-mediated immunity is a central concern for clinicians. Groups at particular risk of progressing from LTBI to active TB disease are HIV-positive individuals and individuals with immune-mediated inflammatory diseases (such as rheumatoid arthritis, Crohn's disease, and ankylosing spondylitis), who are iatrogenically immunosuppressed or treated with TNFα blockade.68 Unfortunately, the TST is well-recognised to have poor sensitivity in these immunocompromised populations; hence, there is a need to evaluate the diagnostic performance of the IGRAs in these populations.
HIV- infected individualsThe evidence base for the performance of the IGRAs in HIV-positive individuals has expanded recently since the first reports from Chapman et al and Liebeschuetz et al in 2002 and 2004, respectively.69,70 Studies examining the diagnostic sensitivity of the ELISpot in HIV-seropositive patients suggest it has a higher sensitivity than the TST.69–73 In a recent study of HIV-positive individuals from a low-prevalence setting, the ELISpot was found to be more sensitive than the TST and to significantly correlate with previous active TB disease, which was not the case for the ELISA or TST.73 In a large prospective study of South African children with suspected TB, the sensitivity of the ELISpot was higher than that of the TST and was unaffected by factors known to adversely affect the sensitivity of the TST: HIV infection, malnutrition, and age under 3 years.70 The overall sensitivity in this study was 83%, rising to 91% when combined with the TST.70 In a study of TB-HIV coinfected adults with smear-positive pulmonary TB in Zambia, the sensitivity of the ELISpot was maintained at 92%.69 Subsequent data from a variety of settings has confirmed that ELISpot results are robust in HIV infection and independent of CD4 cell count.70,72–74 One of the largest of these studies, conducted by Clark et al, found that ELISpot results were independent of CD4 cell count in HIV-positive individuals.75 Similar results were obtained by Dheda et al who showed that in HIV-positive individuals the proportion of indeterminate results was low and independent of CD4 count.74
A number of studies have also evaluated the ELISA in HIV-positive individuals although the data seem to be more variable. A recent study of individuals with active smear-positive TB in Zambia compared ELISA and TST positivity in HIV-positive and negative individuals. The investigators found that the sensitivity of the ELISA in HIV-positive individuals (63%) was significantly lower than in HIV-negative individuals (84%).76 In another cross-sectional study of HIV-infected individuals routinely attending a HIV-clinic in a high-prevalence setting, the ELISA was found to have a lower rate of positivity than either the ELISpot or the TST.71 Conversely, in a Chilean study of HIV-positive adults, the ELISA had a higher positivity rate than the TST and significantly correlated to levels of TB exposure, whilst the TST did not.77 Whereas these investigators did not find any impact of CD4 cell count on ELISA results,77 other authors have found that in HIV-positive individuals with very low CD4 cell counts, the ELISA's performance is adversely affected, as exemplified by a higher proportion of negative and indeterminate results. For example, in a large Danish study of 590 HIV-positive patients, whilst in-tube ELISA results were associated with risk factors for LTBI, there was also a significant association between low CD4 cell count and indeterminate ELISA results.78 Subsequently, a number of studies have also found that indeterminate results increase in severe immunosuppression, a fact that may affect the diagnostic utility of the ELISA in HIV-positive individuals.71,76,78–80
Individuals with immune-mediated inflammatory diseases receiving iatrogenic immunosuppressionTo date, there are relatively few published studies exploring the performance of IGRAs in diagnosing LTBI in individuals with immune-mediated inflammatory diseases (IMID). Although most of the available information is from small studies, it does, nevertheless, provide some indication of the performance of IGRAs in this population. At present the evidence base for the ELISpot is smaller than that for the ELISA; most of the studies carried out are cross-sectional in design and focus on the concordance between the TST and IGRAs rather than correlating surrogate markers of LTBI.17 These studies have reported generally poor or fair concordance between the TST and IGRAs in individuals with IMID, and show that discordant TST+/IGRA- results are significantly associated with prior BCG vaccination.81–86 Recent work from a large Italian study, however, in which the ELISA was used to screen individuals with IMID found relatively high concordance between the TST and ELISA (87.5%, κ=0.55) with a lower proportion of TST+/IGRA- discordant results, which is likely related to the low percentage of the population who had previously been BCG-vaccinated (4.1%).87
In individuals with IMID, fewer studies have correlated the presence of risk factors for LTBI with results of the IGRAs and TST. An Irish study of individuals with inflammatory arthritides reported a significant association between the presence of risk factors for LTBI and positive ELISpot and ELISA results.88 In an Italian study of 398 patients, both ELISA and TST positivity were significantly associated with being in close contact with sputum smear-positive TB patients.87 Similar results were found in a study of 142 patients with IMID, in which a positive ELISA, but not TST, was significantly associated with risk factors for LTBI.81
The general consensus that can be drawn from these studies is that in individuals with IMID receiving immunosuppressive therapy, IGRAs maintain their diagnostic sensitivity better than the TST, but false-negative results are not uncommon.
Specificity of interferon-gamma release assaysQuantifying diagnostic specificity for the IGRAs has been primarily achieved by studying BCG-vaccinated individuals from low-prevalence regions, who are at a low risk of LTBI due to the absence of risk factors for TB exposure. A greater number of studies have evaluated the specificity of the ELISA, reporting a range of 89–100%, with a pooled specificity of 99% for the second generation ELISA and 96% for the latest generation, in-tube ELISA.89 Studies using the ELISpot have also found high diagnostic specificity, which ranges from 85–100%, with a pooled specificity of 93%.89 Overall, the currently available evidence has consistently demonstrated that the IGRAs have a higher specificity than the TST, particularly in BCG-vaccinated populations.
Rates of indeterminate results in clinical practiceAs the IGRAs begin to be more widely used outside the research setting, it is important to consider the reliability of the blood tests in routine clinical use. Both IGRAs are known to suffer from indeterminate results.3 These most commonly arise due to a failed positive control, which usually reflects underlying cellular immune suppression. Data from a wide range of studies suggest that indeterminate results are not infrequent with the ELISA, occurring in between 5 and 40% of cases with the second generation ELISA, although they may be less common with the newer in-tube ELISA.45,49,55,90,91 Indeterminate ELISA results seem to be associated with young (<5 years) and old age (>80 years) and immunosuppression, either due to HIV infection or immunosuppressant medication.91 Conversely, indeterminate results are rarer with the ELISpot, occurring in 0 to 5.4% of tests undertaken.91
Predictive value of interferon-gamma release assays for progression to active TBPreventive treatment for IGRA-positive contacts will only confer clinical benefit if they are at increased risk of progression to active TB compared with IGRA-negative contacts. Only very recently has clinical outcome of this nature begun to emerge from longitudinal studies. To date, 6 studies have been published assessing the predictive value of IGRAs.92–97 A small study from a low-burden region investigated the use of the ELISA in 601 contacts, and found that a significantly higher percentage of untreated household contacts with a positive ELISA progressed to TB disease as compared to contacts with a 5–mm positive TST, with all 6 incident cases ELISA-positive at recruitment.92 More recently, Aichelberg et al explored the prognostic power of a positive ELISA (QuantiFERON-Gold In-tube) result in HIV-positive individuals without active TB at recruitment.97 The investigators found that over a median follow-up period of 19 months, 3/37 individuals with a positive ELISA at baseline went on to develop active TB, whereas 0/738 with a negative ELISA subsequently developed TB.97
A study from Turkey with 908 child contacts and 15 incident cases found that contacts with a positive ELISpot had a significantly increased risk of developing active TB as compared to contacts with a negative ELISpot.93 TB incidence in ELISpot-positive contacts was similar to that in TST-positive contacts, although the ELISpot predicted these from fewer contacts tested.93 However, as per national guidelines, a large percentage of the children in this study received isoniazid chemoprophylaxis, which is likely to have resulted in an underestimation of the incidence rate ratios.
A longitudinal study conducted in a high-prevalence setting in Gambia followed up 2348 household contacts for 2 years.94 The study found that 11/649 ELISpot-positive individuals developed active disease as compared to 14/843 TST-positive individuals; 10/1087 ELISpot-negative and 11/1387 TST-negative individuals developed TB.94 It was concluded that either test could be used in the initial screen for LTBI in contacts, although neither the TST nor the ELISpot predicted subsequent progression to TB disease. Reasons for this lack of prognostic power of both the TST and ELISpot remain unclear but may partly relate to the highly endemic setting in which de novo community transmission outside the household-setting accounts for a substantial proportion of incident TB cases.98
Longitudinal clinical outcome studies have provided the preliminary evidence base that supports the use of IGRAs to target preventive therapy for recent IGRA contacts, and this will enable prevention of a similar number of cases as when using the TST, but necessitates treatment of significantly fewer contacts.
Summary of clinical dataAlthough the field of IGRA research is expanding and evolving rapidly, a number of general conclusions can be drawn from the currently available data (Table 2). Published studies have clearly demonstrated that IGRAs are not confounded by the BCG vaccine and are more specific than the TST in the diagnosis of LTBI.89 When active disease is used as a surrogate marker for LTBI, both tests are more sensitive than the TST, with the ELISpot having a higher sensitivity than the ELISA.89 In children, the ELISpot seems to be superior to the TST, whereas the ELISA appears to have a comparable sensitivity to the TST, although published data are conflicting. In immunocompromised individuals with HIV infection, on the basis of the currently available evidence, the ELISpot is superior to the ELISA in terms of higher sensitivity and lower indeterminate results. In individuals with IMID undergoing immunosuppressive therapy, the ELISpot and ELISA seem to have comparable efficacy.
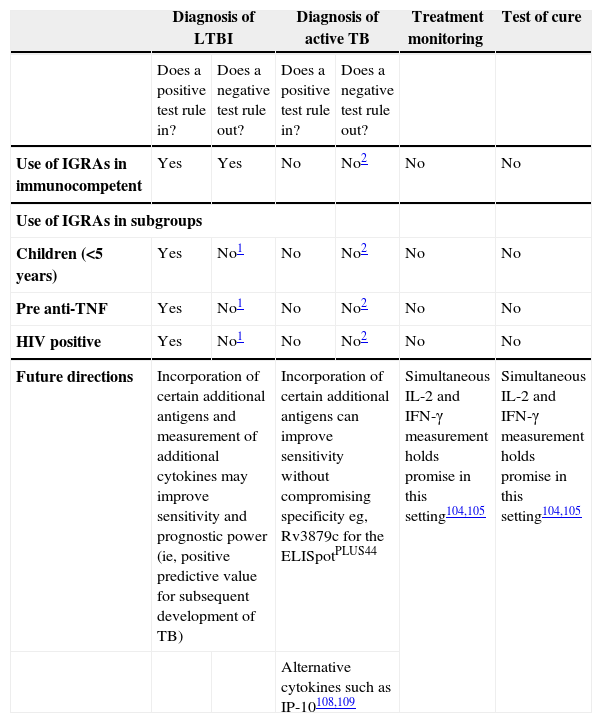
Clinical utility of interferon gamma release assays (IGRAs)
| Diagnosis of LTBI | Diagnosis of active TB | Treatment monitoring | Test of cure | |||
| Does a positive test rule in? | Does a negative test rule out? | Does a positive test rule in? | Does a negative test rule out? | |||
| Use of IGRAs in immunocompetent | Yes | Yes | No | No2 | No | No |
| Use of IGRAs in subgroups | ||||||
| Children (<5 years) | Yes | No1 | No | No2 | No | No |
| Pre anti-TNF | Yes | No1 | No | No2 | No | No |
| HIV positive | Yes | No1 | No | No2 | No | No |
| Future directions | Incorporation of certain additional antigens and measurement of additional cytokines may improve sensitivity and prognostic power (ie, positive predictive value for subsequent development of TB) | Incorporation of certain additional antigens can improve sensitivity without compromising specificity eg, Rv3879c for the ELISpotPLUS44 | Simultaneous IL-2 and IFN-γ measurement holds promise in this setting104,105 | Simultaneous IL-2 and IFN-γ measurement holds promise in this setting104,105 | ||
| Alternative cytokines such as IP-10108,109 | ||||||
Sensitivity of currently-available IGRAs is not yet high enough to rule out suspected LTBI; until next-generation assays of higher sensitivity are available, use of IGRA in parallel with TST may increase overall diagnostic sensitivity.
Diagnostic sensitivity of currently available IGRAs not yet high enough to rule out active TB. Use of TST in parallel can detect some patients with false-negative IGRA results.44
Over the last few years, the IGRAs have gained regulatory approval in the US and Europe and as their evidence base has increased, a number of national guidelines have been rewritten to recommend their use in the diagnosis of LTBI. In Europe and Canada, guidelines advise that the IGRA should be used in 2 situations99,100:
- 1.
As a confirmatory test in individuals who have already tested positive with the TST
- 2.
As a direct replacement for the TST in those individuals in whom the TST is likely to be unreliable (immunocompromised individuals)
Conversely, in the US and Japan, guidelines recommend that the IGRA should completely replace the TST as the test of choice for LTBI in all individuals.101
Economic considerations played a role in the eventual recommendations made by different countries. Although the IGRAs are more expensive than the TST,3 health economic analyses have found that they are cost effective as they reduce the number of individuals needing unnecessary chemoprophylaxis and clinical and laboratory monitoring while on drug therapy.102,103 One such economic analysis, conducted by the United Kingdom National Institute of Health and Clinical Excellence (NICE), concluded that a two-step TST and confirmatory IGRA approach would be most cost-effective strategy, thereby forming the basis of the recommendation made by NICE and subsequently adopted by most other European countries.99
A consequence of the European/Canadian recommendations is that, potentially, immunocompetent individuals with a negative TST who may have been IGRA-positive will not be identified as having LTBI and hence, left untreated. On the other hand, it could be argued that the US/Japanese guidelines imply potential overtreatment of individuals who are IGRA-positive/TST-negative because the risk of progression to active TB in this subgroup is yet to be quantified. This highlights the urgent need for more longitudinal data to quantify the predictive value of positive IGRA results and, in particular, the prognosis of contacts with discordant IGRA and TST results.
Recent developments and future directionsInterferon-gamma release assays are recognised as the 100-year upgrade of the TST for diagnosing LTBI.4 However, it is important to realise that they are ultimately a work in progress with an ever-expanding and evolving clinical and research evidence base. As a result, their advantages and limitations are becoming clearer. Aside from the inability for IGRAs to differentiate between active and latent TB, longitudinal studies involving serial IGRA testing have shown that these tests cannot be used for treatment monitoring or as a test of cure.3 However, there is promising recent evidence that simultaneous profiling of IL-2 and IFN-γ secretion at the single T-cell level correlates with therapeutic response and may be useful in disease monitoring.104,105
While existing IGRAs have higher diagnostic sensitivity than the TST, ongoing research indicates that next-generation assays will have higher sensitivity. One promising approach used for both the ELISA and ELISpot has been to include additional antigens.106 Recent studies have shown that incorporating novel antigens, Rv2645 for the in-tube ELISA107 and RV3879c for the ELISpotPLUS,44 to ESAT-6 and CFP-10 significantly improves sensitivity without reducing specificity.
Current studies are also exploring whether measuring additional, alternative downstream chemokines secreted by IFN-γ-activated macrophages, such as inducible protein 10 (IP-10), monocyte chemoattractant protein (MCP-2) and monocyte inducible protein (MIG), can provide higher sensitivity than the currently available assays which measure only IFN-γ.108 A recent case-control study found that combining IFN-γ and IP-10 significantly increased sensitivity as compared to using the in-tube IFN-γ ELISA alone, without adversely affecting specificity.109 Although these are encouraging results, there is a need for prospective, longitudinal, studies in a range of patient groups (immunocompromised, children, etc.) to establish the accuracy and predictive value of these new IFN-γ and IP-10 tests.
ConclusionsIGRAs are an important new class of diagnostic tool that have the potential to supersede the TST and revolutionise the diagnosis of LTBI. In the few years since their development, the evidence base supporting their use has expanded so rapidly that they now form an integral part of many national guidelines from low-prevalence countries. However, residual uncertainty remains in the differing recommendations framed in these guidelines which reflects the need for large, longitudinal studies to further define the prognostic value of positive IGRA results for development of active TB disease, particularly in high-risk groups and subgroups with discordant IGRA and TST results.
Conflict of Interest StatementProfessor Lalvani is the inventor of patents underpinning T cell-based diagnosis. The Lalvani ELISpot was commercialised by an Oxford University spin-out company (T-SPOT.TB®, Oxford Immunotec Ltd, Abingdon, UK) in which Oxford University and Professor Lalvani have a minority share of equity.