The intracellular penetration and activity of UB-8902 in human polymorphonuclear leukocytes was evaluated. Intracellular UB-8902 concentrations were 6-fold higher than extracellular levels. Uptake was rapid, reversible, saturable, and affected by external pH. UB-8902 showed intracellular activity against Staphylococcus aureus strains presenting mutations associated with fluoroquinolone resistance in the gyrA and/or grlA genes.
Se ha evaluado la penetración intracelular y la actividad de UB-8902 en leucocitos polimorfomucleares humanos. La concentración intracelular de UB-8902 fue más de 6 veces superior a la concentración extracelular. La penetración intracelular fue un proceso rápido, reversible, saturable y no se afectó por el pH del medio. UB-8902 se mostró activo intracelularmente frente a cepas de Staphylococcus aureus que presentaban mutaciones en los genes gyrA y/o grlA, asociadas a resistencia a fluorquinolonas.
The penetration and intracellular activity of antimicrobial agents in phagocytic cells is particularly important in conditions that enable the infecting microorganism to survive and multiply. To treat such infections, it is of clinical interest to use agents able to accumulate and remain active within the cells. A high intracellular accumulation of an antimicrobial agent does not always imply good intracellular activity. Most fluorquinolones are able to concentrate intracellularly in human phagocytic cells and remain active against facultative and obligate intracellular pathogens such as mycobacteria, Legionella spp, Streptococcus pneumoniae, Listeria monocytogenes and Staphylococcus aureus.1–3 Nonetheless, quinolone resistance has steadily risen, and has proven to be associated with mutations, mainly in the gyrA and parC genes.4 The new quinolone UB-8902, a 7-(4-methyl)-piperazine ciprofloxacin derivative, has been designed to avoid these resistance mechanisms. UB-8902 displays good activity against multiresistant microorganisms including Acinetobacter baumannii and Stenotrophomona maltophilia.5 The purpose of this study was to evaluate UB-8902 uptake by human polymorphonuclear leukocytes (PMNs), and investigate the mechanisms this antimicrobial agent uses to penetrate these cells. The intracellular activity of UB-8902 against isogenic Staphylococcus aureus strains presenting fluoroquinolone resistance associated with mutations in the gyrA and/or grlA genes was also evaluated.
Material and methodsIsolation of PMNs. PMNs were recovered from heparinized venous blood of healthy donors, and purified by previously described methods.6 PMNs were adjusted to 5×106cells/mL in Hanks balanced salt solution (HBSS) containing 0.1% gelatine.
UB-8902 uptake by human PMNs. The uptake of UB-8902 (provided by Cenavisa, S.A. Laboratories, Reus, Spain) by human PMNs was determined by a fluorometric assay.6 PMNs were incubated with different concentrations of UB-8902 (1–50μg/mL). After various incubation periods at 37°C, cells were separated from the extracellular solution by centrifugation and the cell pellet was placed in 0.1M glycine-HCl buffer to release the intracellular antimicrobial.
The antimicrobial agent was determined by fluorescence emission of the supernatants measured with a fluorescent spectrophotometer. The fluorescence excitation and emission maxima in 0.1M glycine-HCl were 278 and 447nm, respectively. After determination of the cell volume with radiolabeled C14 polyethylene glycol and H3 water, the accumulation ratio of antimicrobial agent in PMNs was calculated and expressed as the cellular to extracellular concentration (C/E).
Characterization of UB-8902 uptake. The influence of cell viability, environmental temperature (4° vs 37°), pH (5–8), and the metabolic inhibitors sodium fluoride and sodium cyanide 1.5×10−3 M, carbonyl cyanide m-chlorophenyl hydrazone 1.5×10−5 M, and 2,4-dinitrophenol, 1×10−4 M were evaluated. In a series of experiments, UB-8902 uptake by human PMNs was measured after stimulating cells with 200nM phorbol myristate acetate (PMA) and after phagocytosis of opsonized S. aureus (5% pooled human serum at a 10/1 ratio of bacteria to PMN).
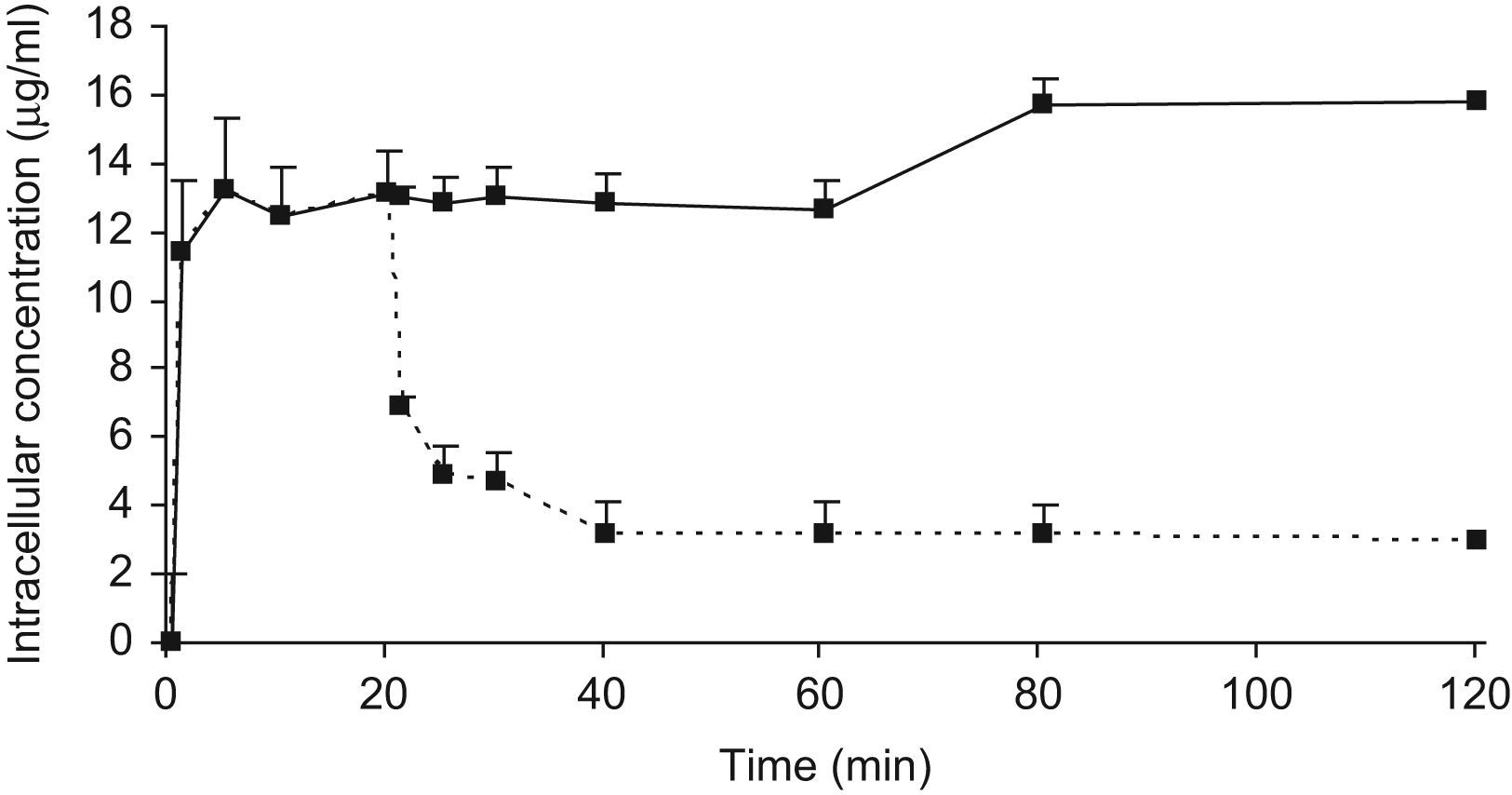
The efflux of PMN-associated UB-8902 was also studied. PMNs were incubated for 20min at 37°C with UB-8902 (2μg/mL), collected by centrifugation, and resuspended in antimicrobial-free medium. Cell-associated UB-8902 was quantified at various time intervals after removal of the extracellular antimicrobial agent.
Organisms and susceptibility testing. Killing assays were performed in S. aureus 5-61 (wild-type), S. aureus 5-61 G8 (grlA mutant; Ser80Phe), and S. aureus 5-61 M14 (gyrA and grlA mutant; Glu88Lys and Ser80Phe, respectively). The minimal bactericidal concentrations (MICs) of UB8902 and moxifloxacin (Hoechst AG) were, respectively, 0.03 and 0.06μg/mL for S. aureus 5-61, 0.125 and 0.5μg/mL for S. aureus 5-61 G8, and 0.5 and 4μg/mL for S. aureus 5-61 M14.
Intracellular activities of antimicrobial agents. The intracellular activity of UB-8902 against mutant and wild-type Staphylococcus aureus in PMNs compared to that of moxifloxacin was evaluated in a 3-h assay using a previously described method.7 Opsonized bacteria and PMN (10:1 bacteria/PMN) were incubated for 60min at 37°C. Extracellular bacteria were removed by differential centrifugation and cells were suspended in RPMI medium. Different concentrations of antimicrobial were then added and cells were reincubated (3h, 37°C). Cells were lysed in distilled water, and the colony-forming units (CFU) were counted on Mueller Hinton agar.
Statistical analysis of data. Data were expressed as the mean±standard deviation. Differences between groups were compared by analysis of variance, which was used to assess statistical significance at a p-value of <.05.
ResultsThe C/E ratio for UB-8902 was 6.5±0.8 after 20min. of incubation at an extracellular concentration of 2μg/mL. When the extracellular antimicrobial had been removed, PMN-associated UB-8902 decreased by 70% after 5min of incubation at 37°C (Fig. 1). Cell-associated UB-8902 was saturable at extracellular concentrations higher than 10μg/mL. The Cmax value previously described for UB-8902 is 7.9μg/mL.
Intracellular penetration of UB-8902 was not affected by death of cells or a temperature of 4°C. PMN uptake of UB-8902 increased significantly at an acidic pH (pH 6; C/E 8.6±1.6). In contrast, sodium cyanide significantly impaired intracellular penetration of UB-8902 (3.3±1.4). Preincubation of PMNs with sodium fluoride, m-chlorophenyl hydrazone, and 2,4-dinitrophenol did not affect UB-8902 uptake; nor did ingestion of opsonized S. aureus cells or cell membrane stimulation with PMA.
The intracellular activity of UB-8902 versus moxifloxacin against mutant and wild-type S. aureus was evaluated by a 3-h assay (Fig. 2). At all the extracellular concentrations evaluated, UB-8902 decreased intracellular survival of mutant and wild-type S. aureus. The intracellular activity of moxifloxacin was slightly higher than that of UB-8902.
DiscussionHigh intracellular accumulation of an antimicrobial agent is required to enable eradication of an infecting microorganism. The intracellular concentration of UB-8902 was 6.5 times higher than the extracellular level, similar to the values reported for ofloxacin, gemifloxacin, and lomefloxacin,2,4,8 and slightly lower than the values for other quinolones such as DX-619, trovafloxacin, and moxifloxacin.7,9,10 UB-8902 uptake was rapid, saturable, and reversible.
Cell viability and environmental temperature had no effect on penetration of UB-8902 into phagocytic cells. PMN uptake of UB-8902 increased significantly at an acidic pH. Of the inhibitors evaluated, only sodium cyanide significantly impaired intracellular UB-8902 penetration. It is interesting that sodium cyanide, an inhibitor of mitochondrial oxidative metabolism, reduced UB-8902 uptake by PMN, as these cells have few mitochondria, being independent of oxidative metabolism for energy. As reported for ofloxacin and its isomers, these data point to a possible passive mechanism involved in human PMN uptake of this quinolone.2 The effects of phagocytosis and cell membrane stimulation on UB-8902 uptake were similar to those previously observed with trovafloxacin and sparfloxacin. Ingestion of opsonized S. aureus cells and cell membrane stimulation with PMA did not modify intracellular penetration of UB-8902.
At all extracellular concentrations evaluated, UB-8902 significantly reduced intracellular survival of the wild-type strain. The reduced intracellular survival of the two mutant strains was only significant at the higher extracellular concentrations evaluated (1 and 5μg/mL). The intracellular activity of UB-8902 against the two mutant strains was similar. These results are comparable to those observed for moxifloxacin. However, moxifloxacin showed slightly higher intracellular activity than UB-8902 against the wild-type and mutant strains studied. This effect could be partly due to greater intrinsic activity and to the higher intracellular accumulation of moxifloxacin compared to UB-8902.
In summary, UB-8902 penetrates human PMNs and attains intracellular concentrations several fold higher than extracellular concentrations. A passive mechanism may be involved in UB-8902 uptake by PMN. At extracellular concentrations greater than 1μg/mL, UB-8902 remains intracellularly active against fluorquinolone-resistant S. aureus strains with mutations in the gyrA and/or grlA genes.
Conflict of interestThe authors have no conflicts of interest to declare.
This work was funded by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III- FEDER, the Spanish Network for the Research in Infectious Diseases (REIPI C03/14 and REIPI RD06/0008) and by the Consejería de Innovación, Ciencia y Empresa de la Junta de Andalucía (CTS-02908).