Multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least isoniazid and rifampin, is an important public health problem.1 The skeletal system can be involved in 1–3% of all tuberculosis cases2; however, there are very few reported cases of skeletal MDR-TB. In addition to the challenges encountered when treating pulmonary MDR-TB, osteoarticular MDR-TB poses additional difficulties, due to the lack of evidence to guide treatment, the unknown efficacy of second-line antituberculous drugs in the bone, and the paucity of reports on this condition. We present a patient with MDR-TB spondylitis with successful response to treatment.
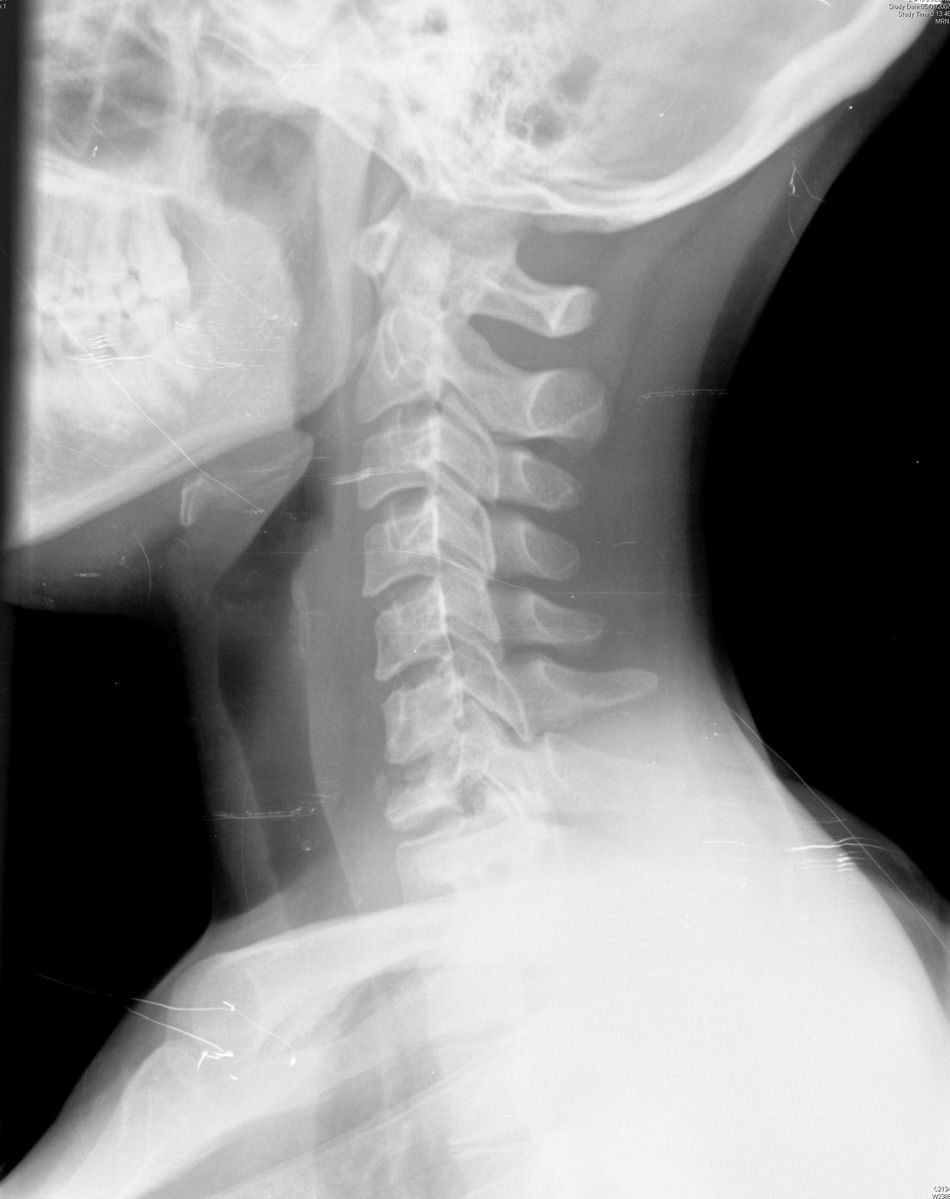
A 32-year-old woman arrived in the emergency department with a neck injury. One year before admission, she started having posterior cervical pain and paresthesias in both hands. On admission, the radiography of the cervical spine showed destruction and collapse of C7 vertebral body (Fig. 1), and a magnetic resonance showed collapse of C7 and oedema of C6, C7, T1, and T2 vertebral bodies. She underwent surgical repair with implantation of C7 vertebral prosthesis for stabilisation. The bone biopsy of C7 showed chronic osteomyelitis and granulomata with central necrosis. The acid-fast stain was negative. The culture yielded Mycobacterium tuberculosis, resistant to isoniazid, rifampicin and pyrazinamide, and sensitive to ethambutol, streptomycin, amikacin, levofloxacin, ethionamide, cycloserine and para-amino salicylic acid.
She was transferred to our tuberculosis unit. On admission, the physical examination was normal, the HIV serology was negative, and the chest X-ray was normal.
She started directly observed treatment with ethambutol 1400mg/day, ethionamide 750mg/day, cycloserine 750mg/day, levofloxacin 500mg/day and intramuscular amikacin 1g every 48h. All drugs were administered for 18 months except amikacin, which was administered for 3 months. There were no adverse effects except polyarthralgias (which improved after substituting moxifloxacin for levofloxacin in the third month). After 18 months of therapy, she had no symptoms and a CT scan showed complete resolution of the vertebral lesions. She declined to continue treatment and she did not return for follow-up. However, we contacted her by telephone 28 months after the end of treatment and she told us she had no symptoms of relapse.
Spondylitis due to MDR-TB is very infrequent: we have only found 6 case reports and two small series3,4 in the literature, with very little information on drug treatment. The optimal treatment for osteoarticular MDR-TB is unknown. The treatment of non-resistant spinal TB is mainly medical, and surgery is reserved for managing complications or failure of antimicrobial therapy.2,5,6 However, the role of medical and surgical therapy in skeletal MDR-TB is not well established, nor is it known what drug combinations would be best to treat this condition. Guidelines for the treatment of MDR-TB have been published,1,7,8 but they give no specific recommendations for osteoarticular MDR-TB.
At the time our patient was treated, we followed the 2008 WHO guidelines,1 which recommended the use of at least four anti-tuberculosis drugs with either certain, or almost certain, effectiveness, given as directly observed therapy for a minimum of 18 months after culture conversion. We used a first-line oral agent (ethambutol), an injectable agent, a fluroquinolone, and two oral bacteriostatic second-line agents (cycloserine and ethionamide), as recommended in the WHO guidelines. Although we did not use it, linezolid is probably worth considering, as it has been extensively used to treat osteoarticular infections and it has good activity against MDR-TB.9,10 In our case, it was not possible to assess culture conversion, and it might have been reasonable to extend the treatment time; however, we decided to stop the treatment after 18 months since the patient did not wish to continue, she was asymptomatic, and there was radiologic evidence of complete recovery of the bone lesions. The 2011 update to the WHO guidelines has recently increased the recommended treatment time to a minimum of 20 months.7