Staphylococcus caprae is a commensal coagulase-negative staphylococci (CoNS) originally isolated from the skin and mammary glands of goats, species in which it causes mastitis.1,2S. caprae is also an infrequent commensal of human skin and has been associated with different syndromes, mainly acute otitis externa, bacteraemia and bone and joint infections (BJIs) in patients carrying orthopaedic devices.1 Herein, we report a case of septic arthritis caused by S. caprae following an arthroscopy for internal meniscus tear repair in a patient without any foreign device implantation.
A 35-year-old healthy male underwent a knee arthroscopy for internal meniscus tear repair, with partial meniscectomy. The procedure was performed uneventfully and no drug, suture or foreign device was used during surgery. Patient was discharged on the same day of surgery. His knee had been operated 17 years before for an osteochondritis dissecans by arthroscopy.
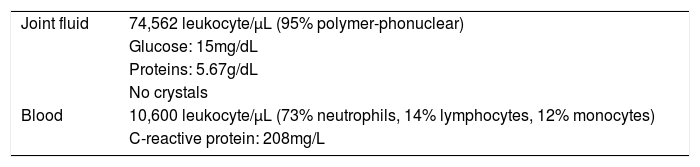
One week after surgery the patient was addressed to the Emergency Department due to severe knee pain and swelling. Neither fever nor other systemic symptoms were observed. Physical examination revealed joint effusion and arthrocentesis yielded 140mL of sero-hematic joint fluid which was sent for analysis and culture. The diagnosis of septic arthritis was established according to the findings of joint fluid and blood analysis (Table 1).
Arthroscopic lavage was performed and intraoperative sample of joint fluid was taken for culture nine days after index surgery. Empiric antimicrobial therapy was started after surgery, with 1g vancomycin IV every 12h and 750mg ciprofloxacin orally every 12h.
Culture of the two joint fluids were processed according to the Infectious Diseases Society of America guidelines and both were positive to S. caprae.3 Gram stain of both samples was negative and the microorganisms only grew in the blood culture bottle and thioglycollate broth sub-cultures after 48h. Identification and antimicrobial susceptibility testing of both isolates was performed by Vitek 2 system (bioMérieux, Marcy l’Etoile, France) and the results were interpreted according to Clinical and Laboratory Standards Institute guidelines.4 The identification was confirmed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, yielding both a 1.74 score (Microflex™; Bruker Daltonik GmbH, Bremen, Germany). The isolates were susceptible to all antimicrobial tested. Five days after surgery, at hospital discharge, an oral regimen of amoxicillin/clavulanic acid 875mg/125mg every 8h was started. Treatment was maintained for 3 weeks, until C-reactive protein (CRP) reached the normal range (3.6mg/L). Clinical evolution was satisfactory, complete range of motion and absence of pain and swell were observed after 3 weeks follow-up from the arthroscopic lavage. In the third-month follow-up CRP levels remained low (0.9mg/L) and the good outcome was confirmed also at the 12-month follow-up.
S. caprae has been reported as a human pathogen since 1983. Nevertheless, until now, only 69 cases of BJIs by this bacteria were described, and just 11 (15.9%) were non-associated to foreign-devices, including five cases of osteitis, two cases of diabetic foot infections and four single cases of arthritis, spondylodiscitis, recurrent osteomyelitis and chronic osteitis.1,2
Septic arthritis after knee arthroscopy is a very uncommon complication with a low incidence rate (0.009–1.1%), mainly caused by S. aureus and CoNS.5,6 Although patients’ skin, can be colonized by S. caprae, the relationship between BJI and nosocomial S. caprae infection is difficult to probe.1,7 Nevertheless, the authors of the only previous report of an intra-articular S. caprae infection following knee arthroscopy suggested that inoculation of the microorganism into the joint could be iatrogenic.8 In this regard, herein we present the second case with similar features in which the hypothesis of patient's skin as the origin of the infection is strongly suspected. In both cases, no device was implanted into the joint of the patient and symptoms appeared one week after primary surgery. S. caprae is usually susceptible to all most antimicrobials for Gram positive bacteria and clinical evolution of our patient was satisfactory with amoxicillin/clavulanic acid treatment.1,8 However, when device-associated S. caprae BJIs occur, foreign device removal is often necessary due to repetitive infection recurrences with more conservative management.1,9 The presence of foreign device and the biofilm formation could facilitate the virulence of S. caprae and explain the poor response to treatment in these cases.9
The study here described contributes to demonstrate once again the ability of S. caprae to develop BJIs, including non-device-associated infections. We would like to highlight the importance of proper skin disinfection before surgical interventions, in order to avoid infections by bacteria from the skin microbiota. Furthermore, the relevance of obtaining deep samples or joint fluid should be remarked, as well as the importance of the inoculation in BC bottle and/or enrichment broth to establish the etiologic diagnosis of this kind of infections.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsOn behalf of all authors, the corresponding author states that there is no conflict of interest.