Syphilis is an infectious disease caused by the spirochaete Treponema pallidum subsp. pallidum which is transmitted by sexual contact or vertical transmission during pregnancy. The incidence of syphilis has increased in the last years, mainly among men who have sex with men. Without treatment, the disease develops into different clinical stages, being able to present cardiovascular or irreversible neurological complications after a number of years. The disease is classified as early syphilis – primary, secondary and early latent syphilis (less than 12 months) – which is contagious, and as late syphilis – late latent and tertiary syphilis – which is rarely contagious. Diagnosis and management are often a challenge because of its diversity of manifestations and the difficulty of interpretation of serological tests. The treatment of syphilis is based on penicillin or doxycycline in allergic patients. Treatment failure because of resistance has been described with azithromycin. The follow up with a serological test is recommended in all patients with syphilis in order to ascertain cure after the treatment and to diagnose possible reinfections.
La sífilis está causada por la espiroqueta Treponema pallidum subsp. Pallidum, que se transmite por vía sexual o vertical durante la gestación. Su incidencia se ha incrementado en los últimos años, especialmente entre los hombres que tienen sexo con hombres. Sin tratamiento, la infección progresa en distintas fases que terminan en complicaciones irreversibles neurológicas y cardiovasculares. Para su clasificación diferenciamos entre sífilis precoz (primaria, secundaria y latente de menos de un año), que es infecciosa, de la sífilis tardía (latente de más de un año y terciaria), en la que el paciente no es contagioso. El diagnóstico y el tratamiento no es sencillo debido a la gran variedad de manifestaciones clínicas y a la dificultad en la interpretación de las pruebas serológicas. El tratamiento de la sífilis se basa en la administración de penicilina o de doxiciclina en casos de alergia. Con azitromicina se han descrito fracasos terapéuticos y se han encontrado resistencias. Los pacientes que hayan sido diagnosticados y tratados deben de ser seguidos para evaluar la respuesta al tratamiento y diagnosticar posibles reinfecciones.
Syphilis is a systemic infection caused by Treponema pallidum (T. pallidum) subsp. pallidum belonging to the Spirochaetaceae family. Other treponematoses that can affect humans are produced by T. pallidum subsp. pertenue, T. pallidum subsp. endemicum and Treponema carateum, which cause yaws, bejel or endemic syphilis and pinta, respectively. All of them are gram-negative bacteria, with a characteristic helical shape, being morphologically indistinguishable from one another. Only T. pallidum subsp. pallidum is transmitted sexually, by oral, vaginal or anal sex, with an infectivity of around 30%. Vertical transmission can occur in the first 4 years after infection with a foetal mortality rate of more than 30–40%.
It is an infection that, without treatment, evolves through various phases. Depending on the time elapsed from infection to diagnosis, it is classified as early syphilis or late syphilis. According to the ECDC,1 early syphilis is syphilis that has been acquired in the last year (2 years according to the WHO)2 and includes primary syphilis (or syphilitic chancre), secondary syphilis (clinically compatible with positive serology) and early latent syphilis (positive serology in asymptomatic patients). Late syphilis is syphilis acquired more than a year previous, and includes late latent syphilis and tertiary syphilis.
For classification it is important to know the clinical, serological and behavioural data (stable partner, number of contacts in recent months, etc.). The diagnosis of early latent syphilis is made when there is a change in serology in the last year (its conversion to positive or increase of 2 dilutions in patients with a history of syphilis) or acquisition after recent contact with a sexual partner diagnosed with early syphilis. Sometimes, due to a lack of information, it is not possible to classify it as early or late latent syphilis, so in these cases it is known as latent syphilis of unknown duration.
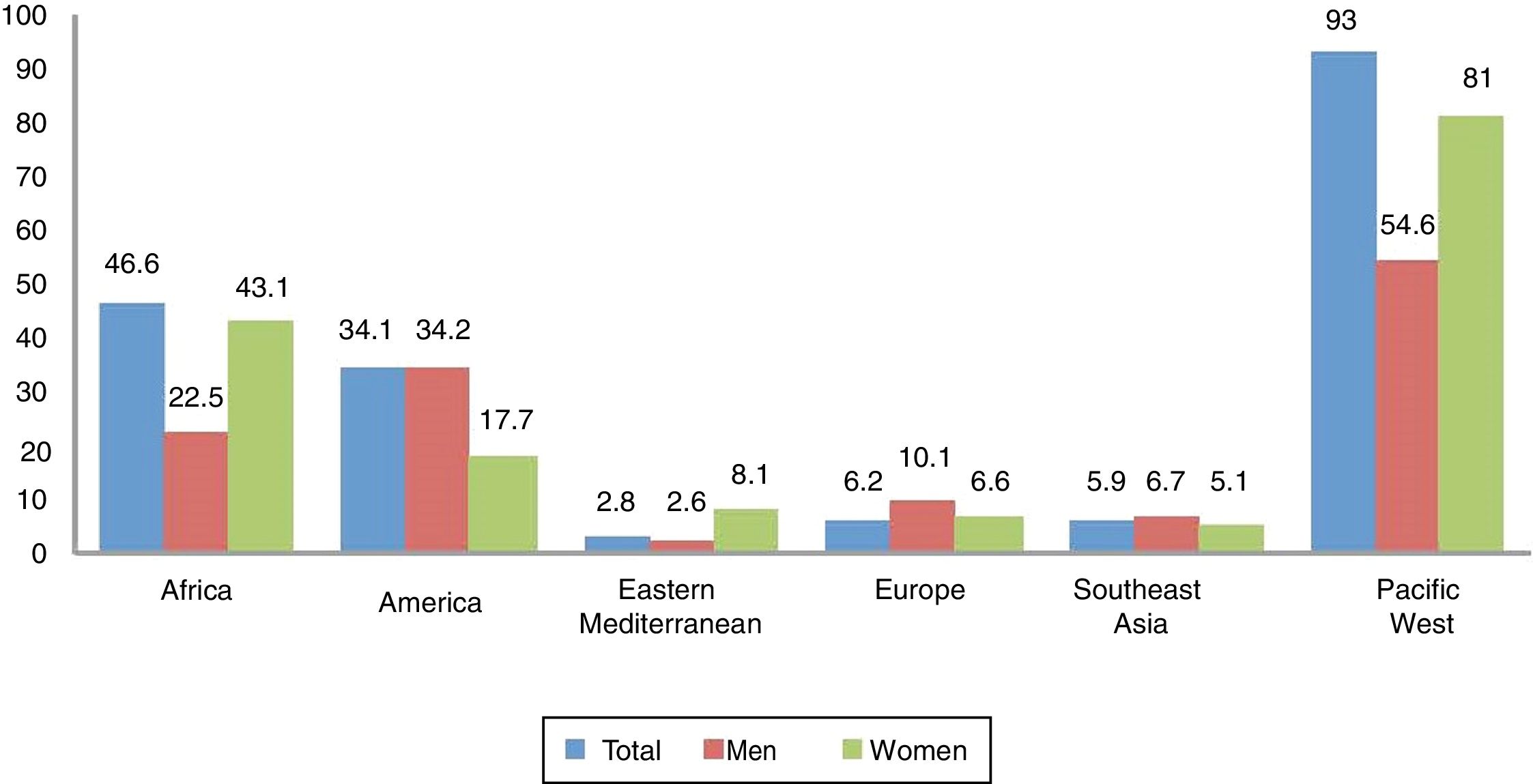
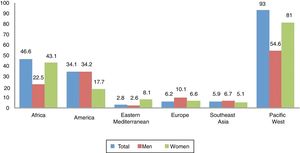
EpidemiologyAccording to the WHO,2 in 2012 there were 5.6 million new cases of syphilis with a rate of 25.1 per 100,000 adults in 2014. Globally, the incidence in women and men was similar, with rates of 17.7 and 17.2, respectively, but if analysed by region (e.g., in Africa and the western Pacific, the rate in women is greater than in men) (Fig. 1).
Rate of syphilis (cases per 100,000 adult inhabitants) by region and sex, reported by 55 countries in 2014.
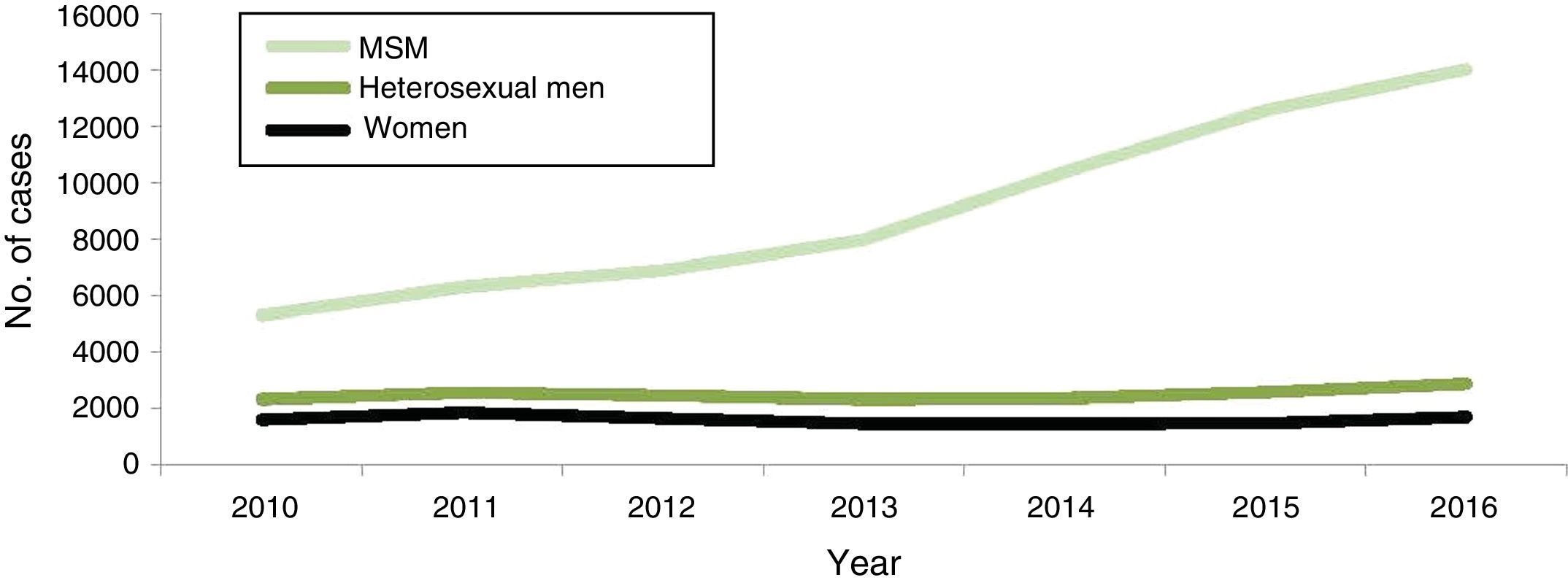
In Europe, the rate of syphilis is higher in men than in women. According to the ECDC1 in 2016, 29,365 cases were reported with an incidence of 6.1 cases per 100,000 inhabitants, a rate that has been increasing since 2011, especially in men who have sex with men (MSM), reaching 66% of the cases reported in this population (Fig. 2).
Evolution of syphilis cases confirmed by gender, transmission groups and year EU/EEA (2010–2016). MSM: men who have sex with men.
In Spain3 in 2016, 3357 new cases of syphilis were reported, with a rate of 7.22 cases per 100,000 inhabitants, affecting men more than women, with a male:female ratio of 9. In that same year, 4 confirmed cases of early congenital syphilis were reported, with an incidence rate of 0.97 per 100,000 live births.
Risk factorsThe increase in cases of syphilis in the last decade has been associated with various risk factors, especially in MSM, such as a greater number of contacts (sporadic or anonymous), contacts made via the Internet or places frequented with the intention of sex (saunas, clubs), drug use and especially having unprotected sex.4–8 These factors are interrelated with each other, and it cannot be clearly determined if they are the direct cause of the infection or an indicator of at-risk behaviour.9
An example of this interrelationship is chemsex,10 a current social phenomenon that occurs mostly among MSM, which is defined as “intentional use of drugs to have sexual intercourse for a long period”, which usually occurs in the context of group sex and in which new information and communication technologies (ICT) play an important role. All these factors have been associated with a greater number of contacts, unprotected anal sex and acquisition of STIs.
Another risk factor that has been described in multiple studies is being HIV positive, which is associated with an increased risk of syphilis. Between 20% and 50% of MSM diagnosed with syphilis are co-infected with HIV11,12 and there are studies where it has been observed that in the 5 years following the diagnosis of syphilis, 10% acquire HIV, with syphilis being a predictor of possible infection of HIV,13–15 in addition to having a greater risk of reinfection of syphilis.13 There are various factors which may explain this association. One of them is that HIV positive patients are diagnosed more frequently by serology (in a latent state)16 when performing periodic laboratory tests. Paradoxically, the use of antiretroviral treatment has been described as a possible cause of the increase in at-risk behaviours (unprotected anal sex) due to a decreased perception of the risk of transmission or acquisition of HIV with undetectable viral loads, with a reduction in the use of condoms and consequently an increase in cases of STIs.17
Clinical manifestationsSyphilis can present in different clinical forms, usually categorised by the duration and location of the infection, although approximately half of infected patients will not develop symptoms and will only be diagnosed by serological tests.
Early syphilisT. pallidum enters the eroded mucosa or skin and begins to divide at the site of inoculation producing primary syphilis. After an incubation period of an average of 21 days (ranging between 9 and 90 days), a single painless papule appears that erodes rapidly, forming an indurated, painless chancre, with a clean base, and firm, raised edges, without pus if there is no superinfection, typically located in the anogenital region (penis, vulva, cervix, perianal). It is accompanied by regional painless nonsuppurative lymphadenopathy. Multiple deep ulcers that can persist over time can be seen in patients infected with HIV.18 Other atypical manifestations include multiple painful, destructive, usually extragenital (most frequently oral) chancres, and sometimes without chancre, as in syphilitic balanitis of Follmann.19
The chancre heals in 3–6 weeks (ranging between 1 and 12) without a trace or if anything, a small atrophic scar, but the treponemes spread throughout the body via the lymphatic system and the blood. The adenopathies may persist longer.
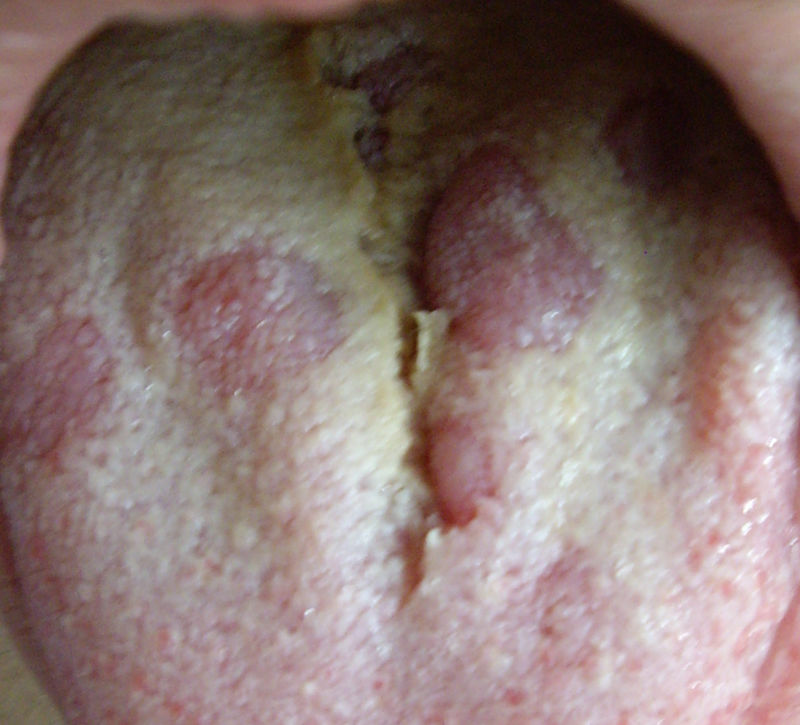
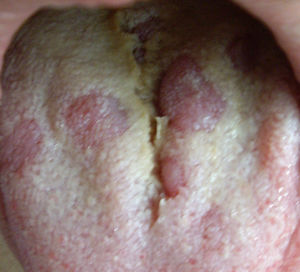
Secondary or disseminated syphilis begins between 2 and 8 weeks after the onset of the initial chancre, which occasionally may still be present. It occurs in 25% of untreated infected individuals. It often presents as a disseminated mucocutaneous rash and generalised lymphadenopathy. The lesions may be macular, maculopapular, papular or pustular, with no vesicular lesions. They usually begin on the trunk or proximal part of the limbs as pink macules, which persist from a few days to 8 weeks, evolving into papules, and in a few patients, pustular lesions. They are usually not pruritic. The different morphologies can be present at the same time and affect any cutaneous location, especially palms of the hands and soles of the feet, which, when affected, strongly suggest the diagnosis. In intertriginous areas, the papules can enlarge, coalesce and erode, forming painless, highly infectious plaques called condylomata lata.20 In the mucosa there may also be highly infectious lesions called mucous patches. In the oral cavity, areas of loss of papillae may appear on the tongue, called plaques en prairie fauchée21 (Fig. 3) and on the scalp areas of alopecia, called moth-eaten alopecia.
In relapses, secondary syphilis lesions are usually less florid, although condylomata lata are common. HIV infection does not alter the clinical symptoms of secondary syphilis.
In secondary syphilis there may be fever, malaise, anorexia and arthralgias. The central nervous system may be affected and in 1–2% of cases aseptic meningitis may develop. In fact, any organ can be affected and glomerulonephritis, hepatitis, synovitis, osteitis, uveitis and others may occur.22
In latent syphilis, which can persist over time, there are no clinical manifestations, only serological reactivity. According to the ECDC1 it is defined as early if it was acquired in the last year, while the WHO2 uses the criterion of 2years. If this temporal criterion is exceeded, or if it is not possible to determine the time of acquisition, it would be considered late latent phase. This differentiation is arbitrary, but it serves the purpose of pointing out that in early latent syphilis secondary relapses are possible (25%). The treatment regimens are also different. In early latent syphilis, sexual transmission of the infection is possible, whereas it is not considered possible in the late latent phase. Vertical transmission is possible in both.
Late or tertiary syphilisThis is a slowly progressive inflammatory disease that can affect any organ and produce clinical disease 20–40 years after the initial infection. It occurs in a third of untreated patients. It is divided into gummatous syphilis (15% of patients), cardiovascular syphilis (10%) and late neurological complications (7%), although nowadays they are rarely seen due to the use of treponemicide antimicrobials for other indications.
Gummata are locally destructive benign granulomatous lesions that can appear up to 40 years after infection, although it is estimated that they appear at 15 years. They can affect any organ, but the most frequent locations are cutaneous (more or less deep nodules) and in the bones (causing fractures, joint affectation). They can be single or multiple and vary in size. Treatment with penicillin achieves a rapid response.
Cardiovascular syphilis occurs 15–30 years after infection. The underlying lesion is endarteritis obliterans that affects the vasa vasorum of the aorta, causing necrosis of the tunica media, with destruction of the elastic tissue, aortitis and formation of an aneurysm, usually in the ascending aorta with valvular involvement.
The term neurosyphilis usually refers to a late stage of the disease, but in reality, and in a broad sense, neurosyphilis is the infection of the central nervous system by T. pallidum and can occur at any time after infection. In early stages it can affect the CSF without clinical signs (asymptomatic meningitis), meninges (symptomatic meningitis) or vessels (meningovascular syphilis), although the latter is seen more frequently in advanced stages. In late disease there is usually overlap of meningovascular and parenchymal involvement. In meningovascular forms, as a consequence of endarteritis obliterans, ischaemic involvement occurs in the brain or spinal cord. In parenchymal involvement there may be cortical neuronal involvement known as general paresis, with impairment of cognitive functions, but tabes dorsalis may also occur, in which the spinal cord undergoes demyelination of the posterior columns and dorsal roots, resulting in the development of an ataxic gait.
Congenital syphilisFoetuses can be infected from any untreated mother, but are more likely to be infected from a mother with early-stage syphilis. Foetal infection before the fourth month is rare, so early abortions are not usually seen. Treatment of the mother during the first 4 months of gestation usually ensures that the foetus is not born infected. In more advanced gestational periods, the consequence of the infection may be abortion, neonatal death, neonatal disease or latent infection. Two thirds of newborns are born asymptomatic, but will develop signs in the following weeks. During the first 2years (early congenital syphilis), the symptoms are usually osteochondral and mucocutaneous, especially rhinitis, which is usually the earliest sign. After 2years, in the late phase, chronic inflammatory lesions similar to gummata develop as well as multiple manifestations: keratitis, deafness, recurrent arthropathy in the knees, frontal protuberance, poorly developed jaws, notched incisors (Hutchinson's teeth) and others.
DiagnosisThe determination of antibodies in serum is the most common form of diagnosis, although direct diagnosis (detection of T. pallidum in lesions, adenopathies, tissues or CSF) is becoming increasingly important thanks to the development of molecular biology techniques. Direct detection provides a definitive diagnosis of syphilis23 and is especially useful in suspicious lesions in serologically non-reactive individuals. The available direct diagnostic techniques are dark-field microscopy and PCR.
Dark-field microscopy of ulcers or exudative cutaneous lesions provides immediate diagnosis by visualising the mobile treponemes. The ideal sample is the serous exudate of active lesions. Blood samples are not valid, and neither are oral nor anal samples. Its main drawbacks are that the observation must immediately follow the collection (<30min), a dark-field microscope and an expert microscopist are necessary, since it is a laborious and subjective method, and that a negative result does not exclude infection.
PCR techniques are displacing dark-field microscopy. They allow the study of extragenital lesions where there may be commensal treponemes, as well as tissues, CSF, vitreous humour or amniotic fluid. They are also of interest in the diagnosis of congenital syphilis and neurolues. They are not recommended in blood because of the existence of inhibitory substances. The samples can be fresh or frozen. CSF PCR is considered to have little value for the diagnosis of neurosyphilis because it has low sensitivity and specificity.
Indirect serological diagnosis provides a presumptive diagnosis23 and does not differentiate syphilis from nonvenereal trepanomatosis (yaws, pinta and bejel).
The non-treponemal (reaginic) tests are the VDRL and the RPR tests. They are manual, simple, cheap techniques that allow for semi-quantification (obtain a titre) but do not allow automation. They are a very useful indicator of disease activity. They change to positive 10–15 days after the appearance of the chancre, which explains their decreased sensitivity in primary syphilis (78–86%), while in secondary syphilis it is 100%. In latent syphilis it remains close to 100% and decreasing to 71–86% in the tertiary phase.
The VDRL is a microflocculation, it must be observed under a microscope (100×) and the acceptable samples are serum and CSF (in the latter contamination with blood or serum should be avoided). The RPR is a macroflocculation that can be assessed without a microscope due to the use of carbon to fix the antigens. It is used in serum and plasma, and its titres are usually higher than those of the VDRL.
These quantitative techniques help to establish the phase of the disease, they can be useful to indicate treatment (titre increase due to reinfection) and also allow for monitoring response to treatment. The fall of the titre after treatment is variable and depends on factors such as the phase of the disease, its duration and the initial titre, but in general it is considered that in early syphilis the titre should fall 4-fold after 6 months. In the case of late syphilis, or after multiple episodes of infection, the fall of the titre is slower and can be stabilised at low titres (VDRL<8, RPR<16). Comparisons of titres between different samples must be carried out in parallel and with the same technique. The persistence of the titre despite correct treatment can occur in patients with autoimmune diseases.
False positives are estimated in 0.2–0.8% of cases and may be transient, usually with low titres, and are observed in pregnancy and in response to conditions that involving polyclonal B-cell activation, such as occurs with immunisations and in acute infections. False positives lasting longer than 6 months usually have high titres and occur in chronic diseases that involve chronic tissue damage (rheumatic fever, myelomas, leukaemias, lymphomas, diabetes, addiction to intravenous drugs and others). There may be false negatives due to the prozone effect (especially in cases of secondary syphilis) and to avoid this, it is advisable to dilute the sera.
Treponemal tests detect specific antibodies against T. pallidum and include TPHA, FTA, immunoblot, EIA and chemiluminescence (CLIA). These are qualitative, more specific and earlier techniques than non-treponemal ones (positive at 1–2 weeks after the chancre appears) and remain positive for life even in treated infections, so they should not be used as markers of activity or for assessment of the success of the treatment. Most detect both IgM and IgG, although it is possible to determine the presence of IgM by specific formats of FTA, immunoblot and EIA, but their sensitivity is low, they do not help to determine the phase of the disease and they would only be useful for the diagnosis of congenital syphilis.
TPHA is a passive haemagglutination that uses erythrocytes sensitised with T. pallidum extract. False positive reactions are possible, but less frequent than in non-treponemal tests. They can occur in the presence of antierithrocyte antibodies, in autoimmune diseases and connective tissue diseases, among others. The FTA test is no longer used in many laboratories because it is very laborious.
In most laboratories of a certain size, the so-called reverse algorithm is used, which uses EIA or CLIA techniques as an initial screening test, due to their high sensitivity and specificity and their automation, which allows many samples to be tested. A negative result excludes infection in the context of screening, while in the context of a patient with diagnostic suspicion the extraction and testing should be repeated at 6 weeks. A positive result occurs both in people with correctly treated prior disease and in untreated patients with active disease, being more sensitive than non-treponemal tests to detect recently acquired syphilis. When a positive initial test is obtained, this must be confirmed with a different treponemal test, usually TPHA, and if this is also positive, a non-treponemal test will be carried out (this can be done on a second sample) to find out the baseline titre that determines the activity and against which the treatment controls will be compared. If the second treponemal test is negative and does not confirm the first, confirmation with an IgG immunoblot can be considered.
The so-called traditional algorithm was used in most laboratories before the appearance of automated tests. It is still used in small laboratories and in countries with few resources. It starts with a non-treponemal test (RPR or VDRL), ideally quantified. It only detects active syphilis. A positive result is confirmed by a treponemal test.
Point-of-care testing (POCT) should be able to provide the diagnosis without the need for additional equipment (refrigerators, centrifuges or microscopes) and has special relevance in the WHO strategy for the elimination of congenital syphilis in countries with scarce resources. In these cases, it has been shown to be cost effective, since it allows diagnosis and treatment to be carried out on the same day. Immunochromatographic techniques are usually used, which are simple to perform using a blood sample obtained by lancet puncture, but which must be performed strictly following the manufacturer's instructions. Formats in which only treponemal antibodies are detected can lead to inappropriate treatments in patients previously infected but treated and cured. There are dual formats that detect both non-treponemal and treponemal antibodies and avoid this problem.24
NeurosyphilisA CSF test should be performed when there is neurological, ophthalmic or otic clinical suspicion, in cardiovascular tertiary syphilis and gummata, and in the face of a therapeutic failure. The definition of asymptomatic neurosyphilis depends on the assessment of the proteins, cells and non-treponemal and treponemal antibodies in the CSF, although there is no consensus definition. The presence of antibodies in the CSF must be assessed together with the clinical symptoms and serves to support the clinical diagnosis, since diagnostic certainty requires histology. It is of utmost importance that the sample not be contaminated with blood. The technique of choice is VDRL (it is more sensitive than the RPR), but neither are very sensitive, so a negative result does not exclude neurolues (false negatives).25 A positive result in the absence of blood contamination is considered indicative of neurolues in late syphilis, but in early syphilis (<1 year) the meaning is not so clear. A negative result from a treponemal test (ideally FTA) in CSF makes neurolues very unlikely, although it does not exclude the diagnosis.26 On the other hand, a positive result may have specificity problems due to the presence of antibodies in CSF as a consequence of alterations of the blood-brain barrier in the context of processes that have nothing to do with syphilis. PCR techniques are also not very helpful due to their low sensitivity and specificity in CSF, although in the absence of a gold standard it is believed that the sensitivity of PCR is underestimated.
Congenital syphilisThe prevention and detection of congenital syphilis depends on the identification of the infection in the pregnant woman. It is essential to investigate any suspicion of active syphilis during pregnancy to ensure proper treatment, monitoring and control of the newborn at birth. In the context of serological pregnancy screening, positive serology for syphilis should be interpreted with caution in light of the serology and previous treatments. If active infection is suspected, in the postpartum serum samples should be obtained in parallel for the mother and the newborn. Cord blood is not valid due to the possibility of contamination with maternal blood. Both treponemal and non-treponemal tests will be carried out in parallel on the samples from the mother and the child, and in the newborn, the presence of specific IgM that can only come from the child will be determined. Positive serology in the neonate usually represents passive transfer of maternal antibodies (not for IgM antibodies), whose titres will decrease with time until they reach undetectable levels between 12 and 18 months. If the titres increase instead of decreasing, or if they remain positive after 18 months, it would indicate congenital infection. Detecting reactive VDRL in the CSF of the newborn also provides a diagnosis. Regarding direct diagnosis, treponemes should be sought in mucosal or cutaneous lesions using dark-field microscopy, immunofluorescence or PCR. Saprophytic treponemes do not appear in the mouth of the newborn until approximately 6 weeks after birth, so the possibility of a false positive is low in dark-field microscopy.27
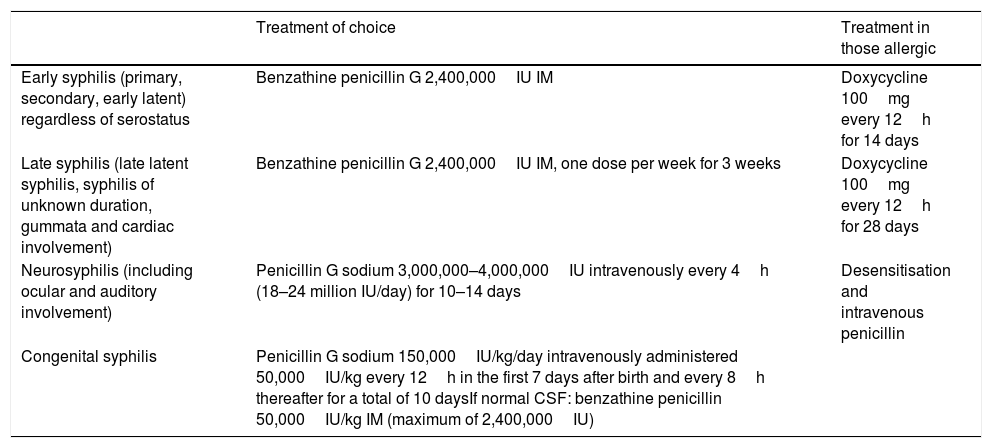
TreatmentThe treatment of choice for syphilis is penicillin. In cases of early syphilis a single dose of 2,400,000IU benzathine penicillin IM, both in HIV positive and negative patients and in cases of late syphilis 3 doses of 2,400,000IU benzathine penicillin IM, one dose per week for 3 weeks (Table 1). Latent syphilis of unknown duration will be treated as late syphilis.
Treatment of syphilis according to stage.
| Treatment of choice | Treatment in those allergic | |
|---|---|---|
| Early syphilis (primary, secondary, early latent) regardless of serostatus | Benzathine penicillin G 2,400,000IU IM | Doxycycline 100mg every 12h for 14 days |
| Late syphilis (late latent syphilis, syphilis of unknown duration, gummata and cardiac involvement) | Benzathine penicillin G 2,400,000IU IM, one dose per week for 3 weeks | Doxycycline 100mg every 12h for 28 days |
| Neurosyphilis (including ocular and auditory involvement) | Penicillin G sodium 3,000,000–4,000,000IU intravenously every 4h (18–24 million IU/day) for 10–14 days | Desensitisation and intravenous penicillin |
| Congenital syphilis | Penicillin G sodium 150,000IU/kg/day intravenously administered 50,000IU/kg every 12h in the first 7 days after birth and every 8h thereafter for a total of 10 daysIf normal CSF: benzathine penicillin 50,000IU/kg IM (maximum of 2,400,000IU) |
In those allergic to penicillin, the treatment of choice is doxycycline 100 every 12h for 2 weeks in early syphilis and 28 days in late syphilis. The efficacy of doxycycline as a treatment has been demonstrated in different studies, without significant differences in therapeutic failure observed in comparative studies between penicillin and doxycycline,28,29 regardless of serostatus. Randomised studies have demonstrated that azithromycin is an effective treatment for early syphilis,30,31 but the description of therapeutic failures and the detection of resistance secondary to 23S rRNA mutation have led to its exclusion from the usual recommendations for the treatment of syphilis.32 The treatment of choice for neurosyphilis (including ocular or auditory involvement) is intravenous penicillin, and desensitisation is recommended in allergic patients. The treatment is the same in HIV negative and positive patients, with no need to increase the total dose in HIV positive patients.
Patients with early syphilis (especially those who have been treated with penicillin), should be warned about the possibility of presenting with the Jarisch–Herxheimer reaction. This reaction presents as a febrile syndrome together with chills, worsening of skin lesions, headache, hypotension, tachycardia, hyperventilation, flushing and myalgias 4–6h after receiving the treatment and normalisation at approximately 24h. Its incidence is approximately 30%, being more frequent in primary and secondary syphilis, with no differences between the two stages.33 In patients with cardiovascular involvement, some experts advise administering corticosteroids before starting treatment to reduce the risk of complications secondary to the Jarisch–Herxheimer reaction.23
Follow-upThere is no definitive test of cure for syphilis. Correct evolution is defined as the disappearance of the symptoms and the decrease of titres of non-treponemal antibodies in 2 dilutions. It is recommended that clinical follow-up be performed at 10–14 days to detect the disappearance of symptoms in patients with primary and secondary syphilis, and perform serological tests on all patients diagnosed with syphilis. Frequency varies according to the diagnosis and the serostatus.34 In HIV negative patients, in early syphilis it is advisable to perform serological tests at 6 and 12 months and in late syphilis at 6, 12 and 24 months. For HIV positive patients, it is advisable to carry out tests at 3, 6, 9, 12 and 24 months, both in early and late syphilis.
If the decrease in 2 dilutions of RPR is not observed at 12 months in early syphilis or at 24 months in late syphilis, lumbar puncture should be performed to rule out possible asymptomatic neurosyphilis.
In the case of neurosyphilis, it is advisable to perform lumbar puncture every 6 months until the cells in the CSF normalise or the VDRL becomes negative.
And as with any recently diagnosed STI, the presence of other STIs should be investigated.
Contact tracingContact tracing differs according to the stage. In case of primary syphilis it is advisable to trace the contacts from the previous 3 months; in the case of secondary, from the previous 6 months; and in early latent syphilis, from the previous 12 months. It is recommended that screening serology be performed on these contacts and, if positive, that they be studied and treated according to the stage. If negative and if the contact was recent (in the previous 3 months), it is advisable to treat as for early syphilis, with a single dose of penicillin or doxycycline for 2 weeks in allergic patients.
In the case of patients with late latent syphilis, it is advisable to carry out a test on the partner and to treat if it is positive.
In patients with latent syphilis of indeterminate duration, in case of having an RPR>1/16, it is advisable to carry out contact tracing as if for late latent syphilis.
FundingThis research has not received any specific funding from public sector agencies, the commercial sector or non-profit organisations.
Conflicts of interestNone.
Please cite this article as: Arando Lasagabaster M, Otero Guerra L. Sífilis. Enferm Infecc Microbiol Clin. 2019;37:398–404.