The objective of this study was to analyse the susceptibility of Mycoplasma genitalium to macrolides and fluoroquinolones using molecular techniques.
MethodsSusceptibility to macrolides was tested (Gipuzkoa, 2014–2017) by a rapid probe-based real-time polymerase chain reaction assay (23S rRNA gene) and to fluoroquinolones by sequencing the parC and gyrA genes.
ResultsMutations associated with macrolide resistance were detected in 43/263 (16.3%) cases and potential fluoroquinolone resistance in 21/267 (7.9%). Macrolide resistance was more frequent in patients previously treated with azithromycin (76.5% vs 7.4%, p<0.001) as well as in those treated with a single 1g dose (31.3%) vs the extended regimen (7%, p<0.001). There were 5/245 (2%) cases with mutations probably associated with resistance to both antibiotics.
ConclusionsThe technique used for testing M. genitalium susceptibility to azithromycin allowed the rapid implementation of resistance-guided antibiotic therapy. Moxifloxacin could be a good option in cases of macrolide resistance.
El objetivo de este trabajo fue analizar la susceptibilidad de Mycoplasma genitalium a macrólidos y fluoroquinolonas mediante técnicas moleculares.
MétodosLa susceptibilidad a macrólidos se analizó (Gipuzkoa, 2014-2017) mediante PCR en tiempo real con sondas (gen 23S ARNr) y a fluoroquinolonas mediante secuenciación tras PCR convencionales (genes parC/gyrA).
ResultadosSe detectaron mutaciones asociadas con resistencia a macrólidos en 43/263 (16,3%) casos y con posible resistencia a fluoroquinolonas en 21/267 (7,9%). La resistencia a macrólidos fue más frecuente tras tratamiento previo con azitromicina (76,5 vs. 7,4%; p<0,001) y con la pauta única de 1g (31,3 vs. 7% pauta ampliada, p<0,001). Se detectaron 5/245 (2%) casos con mutaciones de posible resistencia para ambos antibióticos.
ConclusionesLa técnica empleada para el estudio de la susceptibilidad de Mycoplasma genitalium a la azitromicina permitió una respuesta rápida con un tratamiento antibiótico dirigido. Moxifloxacino puede ser una buena alternativa en casos con resistencia a macrólidos.
Mycoplasma genitalium (M. genitalium) is a major cause of frequently persistent and/or recurrent sexually transmitted infections (STIs).1 The characteristics of this bacterium, with no cell wall and with a small genome, influence the difficulty and slowness (weeks) of isolating it in culture media. Recent development of molecular techniques has allowed diagnosis of this microorganism in clinical laboratories to be put into effect. The treatment regimen currently indicated is azithromycin po 500mg on the first day, extended with 250mg/day for 4 days, using moxifloxacin or 400mg for 7–14 days as an alternative or in cases of recurrence or complicated infection.2
The monitoring of resistance to macrolides in M. genitalium is of special interest, with variable and growing rates being described in some countries (∼15–70%).3,4 This has challenged their use as empirical treatment in urethritis and other non-gonococcal STIs, with some authors recommending doxycycline po 200mg/day for 7 days instead.5 However, the cure rates reported with doxycycline are low (30–40%).1,2
In this situation, and with the antibiotic susceptibility of M. genitalium still little known in Spain,6,7 the objectives of this work have been (1) to analyse the susceptibility of this bacterium to macrolides in Gipuzkoa, using molecular techniques that facilitate the rapid selection of the most appropriate treatment and (2) to determine their susceptibility to fluoroquinolones.
MethodsThe study was conducted between 2014 and 2017 at the Hospital Universitario Donostia [Donostia University Hospital], Gipuzkoa (population served ∼600,000 inhabitants). The samples received for the microbiological diagnosis of patients with suspected STIs (urethritis, cervicitis, asymptomatic contacts, pregnancy screening,...) were analysed daily using a real-time polymerase chain reaction (RT-PCR) technique that simultaneously detects DNA from M. genitalium and 6 other microorganisms (Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma hominis) associated with STIs (Allplex™ STI Essential Assay, Seegene). The samples in which M. genitalium was detected were analysed in under 24h with a second RT-PCR prepared in-house (LightCycler thermal cycler, Roche) that amplifies a 23S rRNA gene fragment and differentiates using probes between wild strains (melting temp ∼59°C) and strains with mutations (positions 2058 and 2059) associated with resistance to macrolides (melting temp ∼50–55°C, supplementary figure).3 The patients received guided treatment (azithromycin or moxifloxacin), follow-up advice for their sexual contacts and they were scheduled for a test of cure at 4–6 weeks.
Subsequently, and to determine the specific mutation in the 23S rRNA gene of the resistant strains, another conventional PCR was performed using the same primers without probes (Thermal Cycler, Applied Biosystems), followed by sequencing (3130XL Genetic Analyser, Applied Biosystems) and comparison of the obtained sequences with that of the standard strain MG37 (BLAST, http://www.ncbi.nlm.nih.gov/blast/Blast.cgi); the duration of these processes was ≥3–4 days. Finally, a fluoroquinolone susceptibility study was carried out using conventional PCRs that amplify one fragment of the parC gene and another of the gyrAgene,8,9 comparing the sequences obtained with that of strain MG37.
ResultsSome 14,167 samples from different locations and infection episodes were analysed in 8388 patients, with DNA from M. genitalium detected in 437 samples from 330 patients (3.9% [95% CI 3.5–4.3%]) 16–67 years of age (median 30 years). Sample or surplus DNA was available for analysis of antimicrobial resistance in 313 patients (202 men, 111 women) who came from primary care (65%, referred to and treated in Microbiology), Gynaecology (21%, 1/3 for pregnancy screening for STIs), STI centre (9%), Urology (2%), and Emergency Departments (3%).
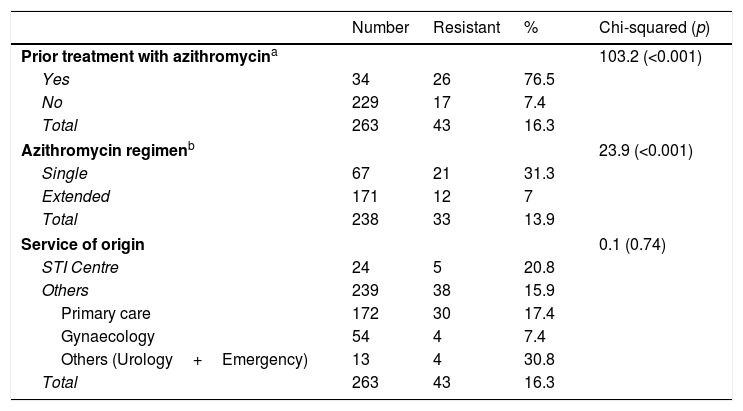
The 23S rRNA gene was successfully amplified in 263/313 patients (84%, 96.1% in the samples with Ct≤35), with macrolide-resistant strains detected in 43 (16.3% [95% CI 12.4–21.3%]) (Table 1). Resistance was greater in men than in women (22.2 vs. 4.6%; p<0.001) and in men who have sex with men than in heterosexual men (32.5 vs. 17.6%; p=0.045) (Table 2). Resistant strains were detected in 17/229 patients (7.4% [95% CI 4.7–11.6%]) who had not previously received previous treatment with azithromycin, although these values were 26/34 (76.5% [95% CI 59.8–87.5%]) among patients with persistent or recurrent infection following the treatment of episodes in which initially sensitive strains were detected (p<0.001) (Table 2). These resistant strains detected following previous treatment with azithromycin represented 60.5% (26/43) of all resistant strains. The development of resistance was also higher in the 21/67 (31.3% [95% CI 21.5–43.2%]) patients in whom the single 1g dose of azithromycin had been used, than in the 12/171 (7% [95% CI 4.1–11.9%]) who had received the extended regimen (p<0.001).
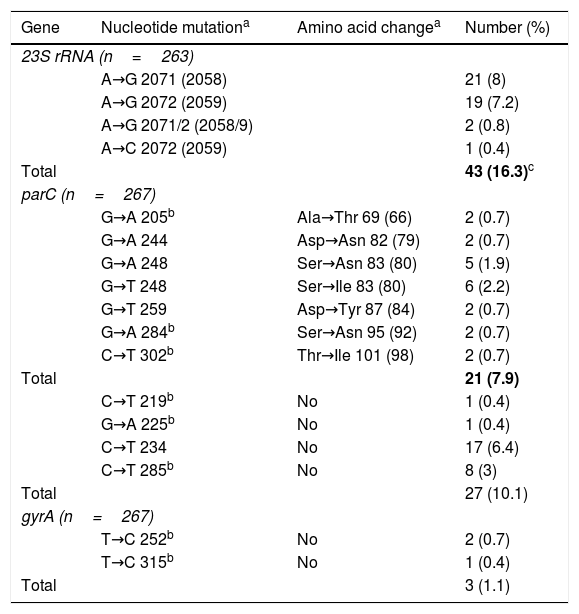
Position and frequency of mutations detected in the 23S rRNA, parC and gyrA genes of Mycoplasma genitalium.
| Gene | Nucleotide mutationa | Amino acid changea | Number (%) |
|---|---|---|---|
| 23S rRNA (n=263) | |||
| A→G 2071 (2058) | 21 (8) | ||
| A→G 2072 (2059) | 19 (7.2) | ||
| A→G 2071/2 (2058/9) | 2 (0.8) | ||
| A→C 2072 (2059) | 1 (0.4) | ||
| Total | 43 (16.3)c | ||
| parC (n=267) | |||
| G→A 205b | Ala→Thr 69 (66) | 2 (0.7) | |
| G→A 244 | Asp→Asn 82 (79) | 2 (0.7) | |
| G→A 248 | Ser→Asn 83 (80) | 5 (1.9) | |
| G→T 248 | Ser→Ile 83 (80) | 6 (2.2) | |
| G→T 259 | Asp→Tyr 87 (84) | 2 (0.7) | |
| G→A 284b | Ser→Asn 95 (92) | 2 (0.7) | |
| C→T 302b | Thr→Ile 101 (98) | 2 (0.7) | |
| Total | 21 (7.9) | ||
| C→T 219b | No | 1 (0.4) | |
| G→A 225b | No | 1 (0.4) | |
| C→T 234 | No | 17 (6.4) | |
| C→T 285b | No | 8 (3) | |
| Total | 27 (10.1) | ||
| gyrA (n=267) | |||
| T→C 252b | No | 2 (0.7) | |
| T→C 315b | No | 1 (0.4) | |
| Total | 3 (1.1) | ||
Differences observed in macrolide resistance rates based on prior treatment with azithromycin, the dose used or the service of origin of the patients.
| Number | Resistant | % | Chi-squared (p) | |
|---|---|---|---|---|
| Prior treatment with azithromycina | 103.2 (<0.001) | |||
| Yes | 34 | 26 | 76.5 | |
| No | 229 | 17 | 7.4 | |
| Total | 263 | 43 | 16.3 | |
| Azithromycin regimenb | 23.9 (<0.001) | |||
| Single | 67 | 21 | 31.3 | |
| Extended | 171 | 12 | 7 | |
| Total | 238 | 33 | 13.9 | |
| Service of origin | 0.1 (0.74) | |||
| STI Centre | 24 | 5 | 20.8 | |
| Others | 239 | 38 | 15.9 | |
| Primary care | 172 | 30 | 17.4 | |
| Gynaecology | 54 | 4 | 7.4 | |
| Others (Urology+Emergency) | 13 | 4 | 30.8 | |
| Total | 263 | 43 | 16.3 | |
The parC/gyrA genes were successfully amplified in 267/313 patients, with mutations potentially associated with resistance detected in 21 (7.9% [95% CI 5.2–11.7%]) and only in the parC gene (Table 1). Of these 21 patients, 16 were successfully treated with azithromycin, but 5/245 (2%) cases with resistance mutations for macrolides and fluoroquinolones were detected. These 5 patients were treated with moxifloxacin (at the time of indicating this treatment, the result of mutations to fluoroquinolones was not known): in 2 cases the infection resolved, 2 cases did not undergo follow-up and in the fifth case M. genitalium was detected again in the test of cure. This patient was treated with doxycycline, after considering reinfection improbable based on contact tracing, and the infection finally resolved.
DiscussionIn this study, in less than 24h after the detection of M. genitalium with a commercial RT-PCR, an additional RT-PCR prepared in-house was indicated and used for differentiation between wild strains (sensitive) and strains with the mutations most frequently associated with resistance to macrolides.3 This speed allowed guided treatment to be carried out, or the empirical regimen to be modified if necessary. Recently, commercial RT-PCRs have been developed that detect the main mutations associated with resistance.10,11
The overall rate of resistance to macrolides in M. genitalium detected in Gipuzkoa was 16.3%. This figure is in the range of those reported in other studies carried out in the general population (14–38%),3,12,13 but is lower than the one described in patients who attend STI centres (35–72%)4,6,9–11,14 (supplementary table). This difference may be due to increased antibiotic pressure on macrolides due to the greater number of STIs treated with azithromycin in patients treated at STI centres, especially men who have sex with men (Table 2 and supplementary table). The resistance observed in the present study better reflects the rate in the general population, since 65% of the patients came from primary care and <10% from an STI centre.
Primary resistance to macrolides (without recent treatment) was only 7.4%, whereas secondary resistance in patients with previous treatment with azithromycin in initially sensitive strains was 76.5% (60% of the resistances detected). This indicates that the mutation responsible for the resistance is induced during treatment and/or that initially minority resistant strains are selected for (in 3/43 patients sensitive and resistant strains were detected in the same sample),3,11,15 although we cannot rule out reinfection by a resistant strain, which occurs in some cases. On the other hand, it has been described that the rates of macrolide resistance in M. genitalium infections treated after a single gram dose are higher than those detected after the extended regimen (0.5g or 1g on day 1, 250mg/day on days 2–5),2 and the results of this study clearly support this. Given this difference and the low clinical response reported with doxycycline,1,2 the results of this study support guided treatment (<24h) following an analysis of susceptibility to macrolides in these infections.
The alternative treatment to macrolides recommended for STIs caused by M. genitalium is fluoroquinolones.2 In this study, mutations potentially associated with resistance in the parC gene were detected in 7.9% of patients, with figures of 3–29% having been described in other studies (supplementary table).6,8–10,12,14 Unlike mutations in the 23S rRNA gene and resistance to macrolides,15 the mutations described in quinolone resistance-determining regions (QRDR) of the parC and gyrA genes in M. genitalium, do not always have an adequate concordance with the clinical response, some differences have been reported depending on the mutation involved and the bacterial load.14 In this study, only 5/245 (2%) patients presented with mutations for resistance to macrolides and quinolones, 2/3 having resolved clinically with treatment with moxifloxacin and possibly also the 2 that did not attend the follow-up.
In conclusion, the results of this study highlight the importance of the detection of M. genitalium in STIs and the benefit of analysing their susceptibility to macrolides with rapid techniques. The rate of resistance to macrolides (16%, 7% in patients without prior treatment) in the general population was lower than that reported in studies performed on patients treated at STI centres. For this STI it is important to perform a test of cure given the frequent detection of mutations during treatment in cases of recurrence. In these cases, moxifloxacin is a good alternative, with a rate of mutations with possible resistance detected of less than 10%, with this strategy allowing treatment with doxycycline to be reserved for the few cases with resistance to macrolides and clinical failure to fluoroquinolones.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Piñeiro L, Idigoras P, de la Caba I, López-Olaizola M, Cilla G. Tratamiento antibiótico dirigido en infecciones por Mycoplasma genitalium: análisis de mutaciones asociadas con resistencia a macrólidos y fluoroquinolonas. Enferm Infecc Microbiol Clin. 2019;37:394–397.