Antimicrobial resistance in Enterobacteriaceae is increasing worldwide and is making treating infections caused by multidrug-resistant Enterobacteriaceae a challenge. The use of β-lactam agents is compromised by microorganisms harboring extended-spectrum β-lactamases (ESBLs) and other mechanisms of resistance. Avibactam is a non β-lactam agent that inhibits clinically relevant β-lactamases, such as ESBL and AmpC. The ceftazidime•avibactam combination (CAZ-AVI) was recently approved for use in certain complicated infections, and may provide a therapeutic alternative for infections caused by these microorganisms.
MethodsThe in vitro activity of CAZ and CAZ-AVI (AVI at a fixed concentration of 4mg/L) was tested against 250 clinical isolates of Enterobacteriaceae using broth microdilution. EUCAST breakpoint criteria were used for CAZ, and FDA criteria for CAZ-AVI. Clinical isolates included bacteria producing extended-spectrum β-lactamases (ESBLs) and acquired AmpC β-lactamases (AACBLs). Porin loss in Klebsiella pneumoniae was also evaluated.
ResultsThe combination of AVI with CAZ displayed excellent activity against clinical isolates of ESBL-producing Escherichia coli and Klebsiella pneumoniae, rendering all the ceftazidime-resistant isolates susceptible to ceftazidime. CAZ-AVI retained activity against porin-deficient isolates of K. pneumoniae producing ESBLs, AACBLs, or both, although MIC values were higher compared to porin-expressing isolates. CAZ-AVI rendered all the ceftazidime-resistant AACBL-producing Enterobacteriaceae tested susceptible to ceftazidime.
ConclusionCAZ-AVI showed potent in vitro activity against clinical isolates of Enterobacteriaceae producing ESBLs and/or AACBLs, including K. pneumoniae with loss of porins.
La resistencia antibiótica en enterobacterias está en aumento y el tratamiento de infecciones producidas por enterobacterias multirresistentes supone un reto terapèc)utico. El uso de betalactámicos se afecta con la producción de betalactamasas de espectro extendido (BLEE) y otros mecanismos de resistencia. Avibactam es un compuesto no betalactámico que inhibe betalactamasas como BLEE o AmpC. La combinación ceftazidima-avibactam (CAZ-AVI) ha sido aprobada recientemente para el tratamiento de infecciones complicadas y puede ser una alternativa terapèc)utica en estas infecciones.
Mèc)todosLa actividad in vitro de CAZ y CAZ-AVI (AVI, concentración fija de 4mg/mL) fue determinada en 250 aislamientos clínicos de enterobacterias mediante microdilución en caldo. Los puntos de corte de EUCAST fueron utilizados para CAZ, y los criterios de FDA se utilizaron para CAZ-AVI. Las enterobacterias estudiadas producían BLEE y/o AmpC adquiridas (BLAA). El papel de la pèc)rdida de porinas en Klebsiella pneumoniae tambièc)n fue evaluado.
ResultadosCAZ-AVI demostró una excelente actividad en Escherichia coli y Klebsiella pneumoniae productoras de BLEE, devolviendo la sensibilidad a CAZ en todos los aislamientos resistentes a CAZ. CAZ-AVI mantuvo su actividad en aislamientos de K. pneumoniae deficientes en porinas productoras de BLEE y/o BLAA, aunque los valores de CMI fueron más altos comparados con las cepas que expresaban porinas. En todas las enterobacterias resistentes a ceftazidima productoras de BLAA analizadas en este estudio CAZ-AVI devolvió la sensibilidad a ceftazidima.
ConclusiónCAZ-AVI demostró una potente actividad in vitro en aislamientos clínicos de enterobacterias productoras de BLEE y/o BLAA, incluyendo K. pneumoniae con pèc)rdida de porinas.
Infections caused by multidrug-resistant gram-negative bacteria are increasing worldwide and pose a therapeutic challenge in clinical practice because treatment choices are limited.1
Extended-spectrum β-lactamase (ESBLs) enzymes with the ability to hydrolyze and create resistance to oxyimino-cephalosporins and aztreonam appeared after the introduction of broad-spectrum cephalosporins.2 The current incidence and prevalence of different ESBLs is a matter of great concern, limiting the therapeutic use of β-lactams.3 In addition, other plasmid mediated β-lactamases such as acquired AmpC beta-lactamases (AACBLs), which prevent the action of cephalosporins, have also spread in recent years. Resistance due to AACBL enzymes is less common than ESBL production in most parts of the world, but may be broader in spectrum.4
With respect to porins, the periplasmic concentration of the β-lactam agent is a function of the permeability of the outer membrane; in particular, the porin channels through which the β-lactams penetrate may play an essential role and contribute to the level of susceptibility to certain β-lactams.5
The use of carbapenems as drugs of choice for the treatment of infections caused by the microorganisms mentioned above has facilitated the appearance and dissemination of carbapenemase-producing Enterobacteriaceae. Alternative agents to carbapenems are needed. In recent years, there has been an alarming decline in the research and development of new antibiotics to deal with the threat of antimicrobial resistance. In 2015, the FDA (Food and Drug Administration, USA) approved the use of ceftazidime•avibactam for the treatment of complicated intra-abdominal infections (in combination with metronidazole) and complicated urinary tract infections in adults including pyelonephritis.6 Ceftazidime•avibactam is also under clinical development for treatment of nosocomial pneumonia, including ventilator-associated pneumonia (VAP) in a phase III clinical trial (NCT01808092).7
Ceftazidime•avibactam may improve the outcome of patients infected with multidrug-resistant gram-negative bacteria. Avibactam is a member of a new class of β-lactamase inhibitors, diazabicyclooctanes (non β-lactam compounds), that inhibit serine β-lactamases, including class A (KPC), class C (AmpC), as well as some class D enzymes (OXA-48). It binds covalently and reversibly to these β-lactamases, so preventing their action.8,9
The aim of this study was to evaluate the activity of avibactam in combination with ceftazidime against a well-defined collection of Enterobacteriaceae producing ESBLs or AACBLs. The role of porin loss was evaluated in Klebsiella pneumoniae isolates.
Material and methodsBacterial strainsA total of 250 bacterial isolates were studied. Species identification was confirmed using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Bremen, Germany).
The strains were selected from four collections:
- a)
ESBL-producing Escherichia coli, isolated during a nationwide study performed in Spain, including the most prevalent ESBLs and E. coli ST131 (n=50).10 In this group, the isolates were not clonally related by REP-PCR (Repetitive Element Palindromic-PCR), with the exception of four isolates with identical REP-PCR profiles that belonged to the ST131 clone and produced CTX-M-15. Thirty-seven isolates were CTX-M producers, including eighteen from the M-1 group (CTX-M-1 n=2, CTX-M-3 n=1, CTX-M-15 n=9, CTX-M-32 n=5 and CTX-M-57 n=1) and nineteen from the M-9 group (CTX-M-9 n=6, CTX-M-14 n=12 and CTX-M-27 n=1) and ten isolates produced SHV-12, and three TEM-type ESBLs (TEM-4 n=1 and TEM-52 n=2).
- b)
ESBL-producing K. pneumoniae isolated in a nationwide study performed in Spain, including the most prevalent ESBLs and clones (n=50).11 They comprised twenty-two CTX-M producers: fourteen from the M-1 group (CTX-M-1 n=4, CTX-M-15 n=9 and CTX-M-32 n=1), six from the M-9 group (CTX-M-9 n=2, CTX-M-14 n=3 and CTX-M-16 n=1) and two CTX-M-type that were not sequenced. Thirteen isolates produced SHV-type ESBLs (SHV-2 n=4, SHV-5 n=2, SHV-12 n=6 and SHV-54 n=1) and eight TEM-type (TEM-3 n=1, TEM-4 n=3, TEM-15 n=1, TEM-25 n=1, TEM-52 n=1 and TEM-133 n=1). Seven isolates produced two ESBLs, five presented one CTX-M-type (CTX-M-1 n=2, CTX-M-14 n=1, CTX-M-15 n=1 and CTX-M-type not sequenced n=1) and SHV-12, and two carried one CTX-M-type plus one TEM-type. (CTX-M-15 plus TEM-74 and CTX-M-type not sequenced plus TEM-3). The 50 isolates showed 45 different REP-PCR profiles.
- c)
K. pneumoniae strains were grouped according to their outer membrane protein profile (POR+/∧) and their ability to produce AACBLs (AACBL+/∧) and ESBLs (ESBL+/∧) (n=49).12 Isolates classified as POR∧ lacked both outer membrane proteins, OmpK35 and OmpK36. Most isolates POR+ lacked OmpK35.
- d)
AACBL-producing Enterobacteriaceae isolated in a nationwide study performed in Spain in 2009. These included the most prevalent species and enzymes (n=100).13,14 As determined by PFGE (Pulsed field gel electrophoresis), the selected isolates were not clonally related.
The minimum inhibitory concentration (MIC) values of ceftazidime (0.015•32mg/L), with and without avibactam (at a fixed concentration of 4mg/L) were determined by microdilution assay in Mueller•Hinton II broth (Oxoid, Spain) following CLSI guidelines.15E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27583 and K. pneumoniae ATCC 700603 were used as reference strains for quality control of in vitro susceptibility testing. Ceftazidime was purchased from Sigma Aldrich (St. Louis, MO, USA) and avibactam was provided by AstraZeneca (Cheshire, England, UK). The interpretation of ceftazidime susceptibility was determined according to EUCAST criteria (susceptible, MIC≤1mg/L).16 The FDA-approved breakpoint for Enterobacteriaceae was used for ceftazidime•avibactam (susceptible, MIC≤8mg/L), based on pharmacokinetic/pharmacodynamic analysis.6
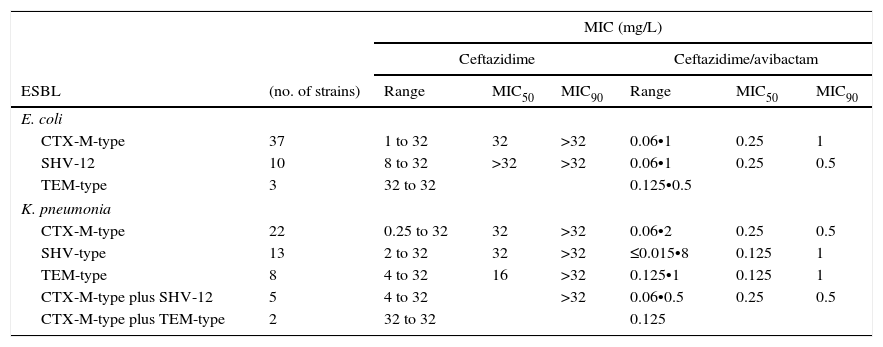
ResultsThe results of the ESBL-producing E. coli group using EUCAST breakpoint criteria for ceftazidime (resistant if MIC>4mg/L) showed that 78% of isolates were resistant, 20% intermediate and 2% (one isolate producing CTX-M-9) susceptible. All isolates were susceptible to the ceftazidime/avibactam combination, displaying MIC values of ≤1mg/L. The MIC range and MIC50 and MIC90 values revealed no differences in the ability of avibactam to protect ceftazidime from hydrolysis by different types of ESBL, as shown in Table 1. The four ST131 isolates showed MIC values for ceftazidime ranging from 4mg/L to >32mg/L, whereas in combination with avibactam they ranged from 0.25mg/L to 1mg/L.
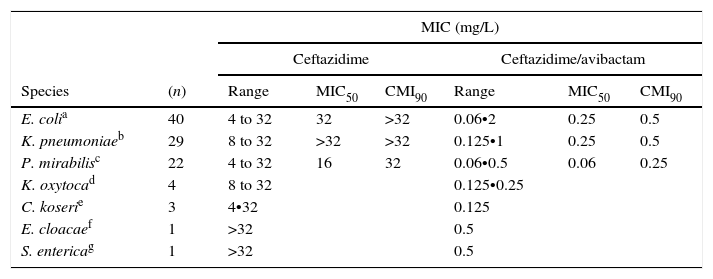
MIC range, MIC50 and MIC90 of ceftazidime and cetazidime/avibactam for 50 ESBL-producing E. coli and 50 ESBL-producing K. pneumoniae, grouped by type of ESBL produced. ESBL: Extended-Spectrum Beta-lactamase.
| MIC (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Ceftazidime | Ceftazidime/avibactam | ||||||
| ESBL | (no. of strains) | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 |
| E. coli | |||||||
| CTX-M-type | 37 | 1 to 32 | 32 | >32 | 0.06•1 | 0.25 | 1 |
| SHV-12 | 10 | 8 to 32 | >32 | >32 | 0.06•1 | 0.25 | 0.5 |
| TEM-type | 3 | 32 to 32 | 0.125•0.5 | ||||
| K. pneumonia | |||||||
| CTX-M-type | 22 | 0.25 to 32 | 32 | >32 | 0.06•2 | 0.25 | 0.5 |
| SHV-type | 13 | 2 to 32 | 32 | >32 | ≤0.015•8 | 0.125 | 1 |
| TEM-type | 8 | 4 to 32 | 16 | >32 | 0.125•1 | 0.125 | 1 |
| CTX-M-type plus SHV-12 | 5 | 4 to 32 | >32 | 0.06•0.5 | 0.25 | 0.5 | |
| CTX-M-type plus TEM-type | 2 | 32 to 32 | 0.125 | ||||
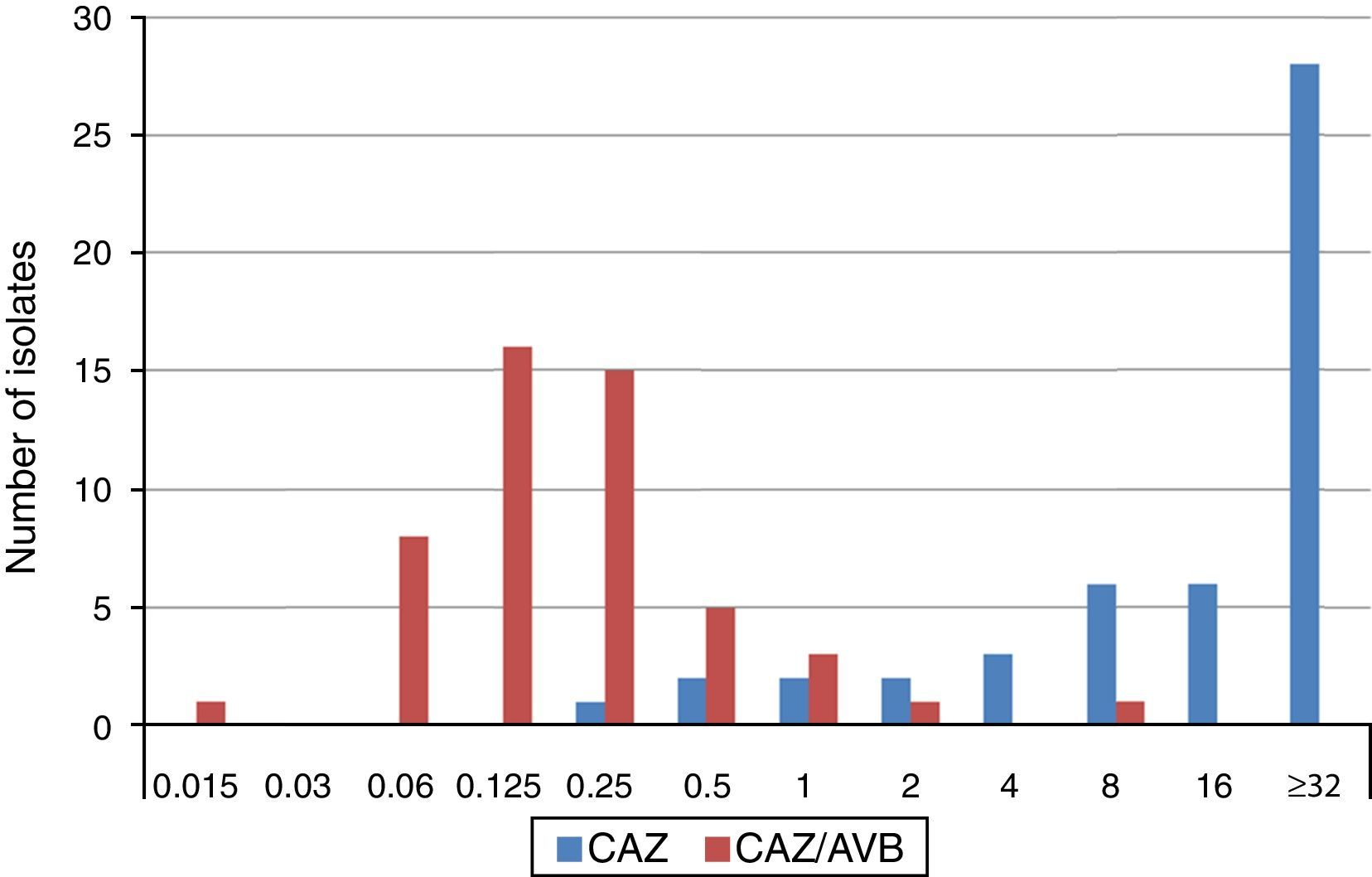
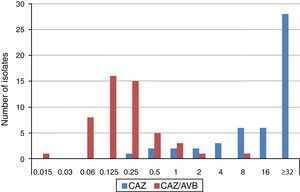
The MIC distributions of ceftazidime, alone and in combination with avibactam, against ESBL-producing K. pneumoniae isolates are shown in Fig. 1. Applying EUCAST breakpoint criteria for ceftazidime, 80% of isolates were resistant, 10% intermediate and 10% susceptible, (five isolates producing CTX-M-type ESBLs, (CTX-M-9 n=1, CTX-M-14 n=3 y CTX-M-type not sequenced n=1) resulting in MIC50 and MIC90 values of ≥32mg/L. The values of the ceftazidime/avibactam combination ranged between 0.125•0.25mg/L and 0.5•1mg/L, respectively. MIC range, and MIC50 and MIC90 values (when applicable) by type of ESBL produced are shown in Table 1. All but two isolates displayed ceftazidime/avibactam MIC values of ≤1mg/L. The two isolates displaying the highest ceftazidime/avibactam MICs included one producing CTX-M-1, with ceftazidime and ceftazidime/avibactam MICs of >32mg/L and 2mg/L, respectively, and another producing SHV-12, with ceftazidime and ceftazidime/avibactam MICs of 32mg/L and 8mg/L, respectively.
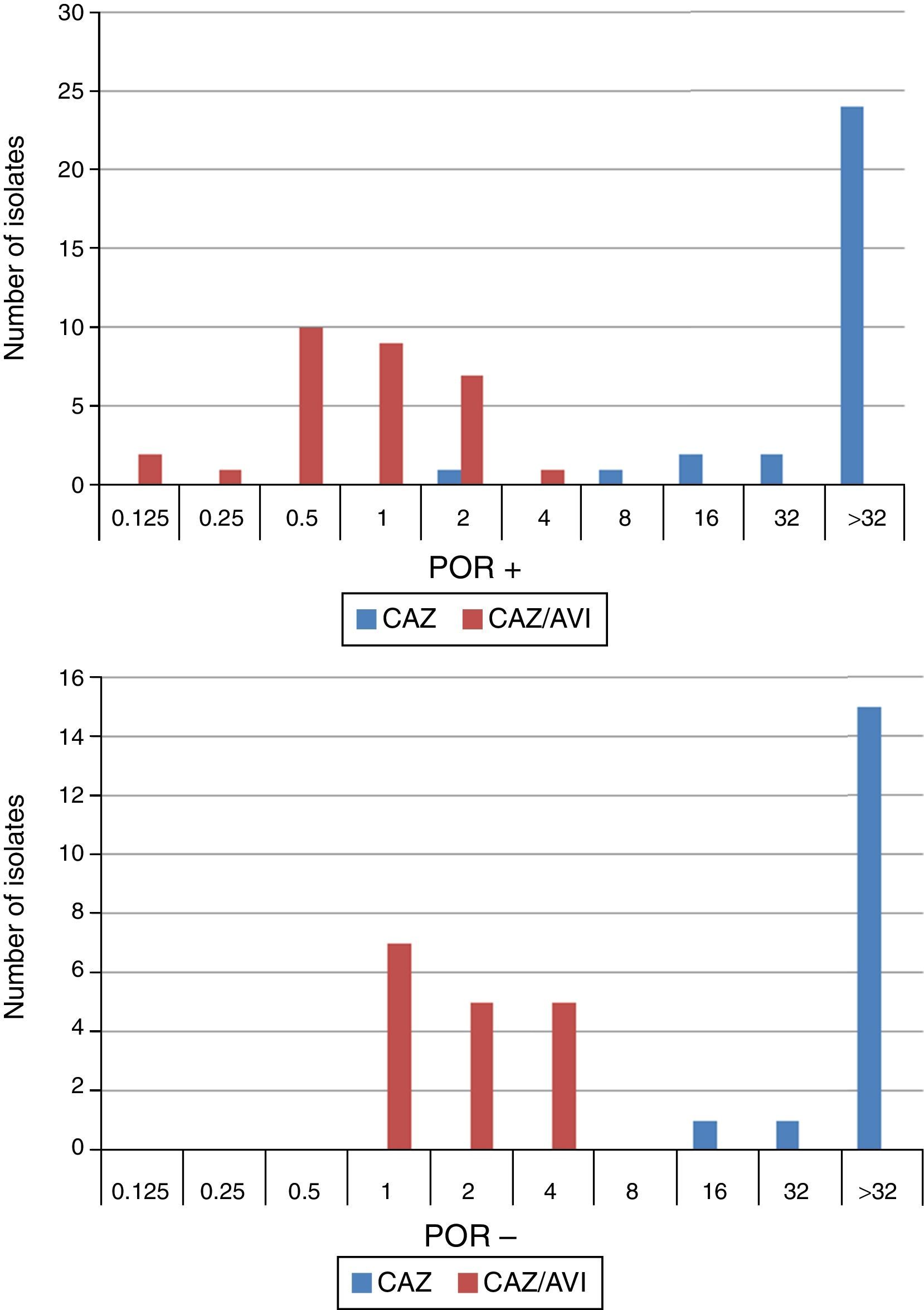
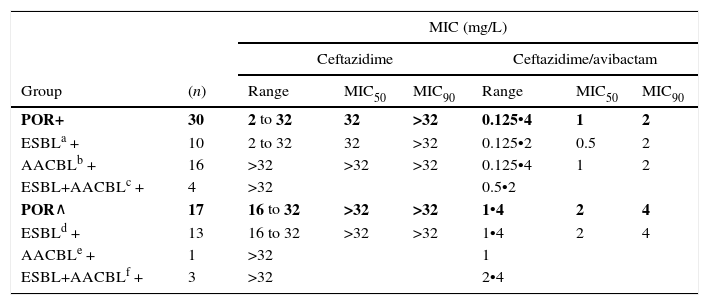
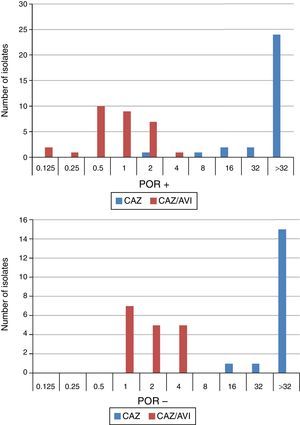
When tested against two POR-, ESBL-, AACBL-K. pneumoniae isolates, the results showed, as expected, that avibactam did not improve the activity of ceftazidime (MIC ceftazidime, 1mg/L; MIC ceftazidime/avibactam, 0.5•1mg/L). The MIC range, MIC50 and MIC90 values (when applicable) of ceftazidime and ceftazidime/avibactam for the 47 isolates producing ESBLs, AACBLs or both, according to porin profile, are shown in Table 2. The MIC distributions of these antimicrobials are shown in Fig. 2. Applying the EUCAST breakpoint criteria for ceftazidime, 47 isolates were resistant to this cephalosporin, with MIC50 and MIC90 values of >32mg/L. The addition of avibactam reduced the MIC50 and MIC90 values in the POR+ group to 1mg/L and 2mg/L, respectively. In the POR- group, the MIC50 and MIC90 values were 2mg/L and 4mg/L, respectively. In both POR+ and POR∧ groups, all isolates were susceptible to ceftazidime when avibactam was added. MIC values for ceftazidime/avibactam of ≤0.5mg/L were observed only for POR+ isolates. All but one of the isolates that displayed the highest MIC value of 4mg/L for this combination were POR∧.
MIC range, MIC50 and MIC90 of ceftazidime and ceftazidime/avibactam for 47 K. pneumoniae isolates producing ESBL, AACBL or both grouped on the basis of the beta-lactamase(s) produced and the porin profile.
| MIC (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Ceftazidime | Ceftazidime/avibactam | ||||||
| Group | (n) | Range | MIC50 | MIC90 | Range | MIC50 | MIC90 |
| POR+ | 30 | 2 to 32 | 32 | >32 | 0.125•4 | 1 | 2 |
| ESBLa + | 10 | 2 to 32 | 32 | >32 | 0.125•2 | 0.5 | 2 |
| AACBLb + | 16 | >32 | >32 | >32 | 0.125•4 | 1 | 2 |
| ESBL+AACBLc + | 4 | >32 | 0.5•2 | ||||
| POR∧ | 17 | 16 to 32 | >32 | >32 | 1•4 | 2 | 4 |
| ESBLd + | 13 | 16 to 32 | >32 | >32 | 1•4 | 2 | 4 |
| AACBLe + | 1 | >32 | 1 | ||||
| ESBL+AACBLf + | 3 | >32 | 2•4 | ||||
AACBL: Adquired AmpC type Beta-lactamase; ESBL: Extended-Spectrum Beta-lactamase; POR: Porin expression. (Isolates classified as POR∧ lacked both outer membrane proteins, OmpK35 and OmpK36. Most isolates POR+ lacked OmpK35).
Results for POR+/POR∧ in bold characters.
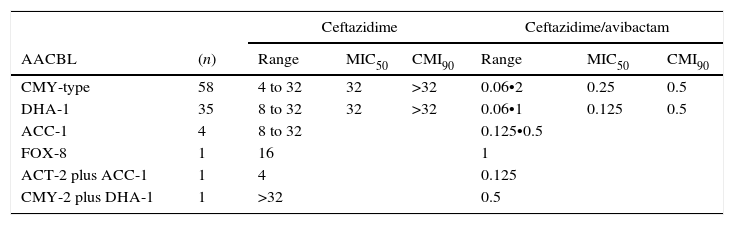
The results for AACBL-producing Enterobacteriaceae, demonstrated that all isolates were resistant to ceftazidime, with MIC50 and MIC90 values of 32mg/L and ≥32mg/L, respectively. The addition of avibactam significantly improved the activity of ceftazidime in E. coli, lowering the MIC50 and MIC90 values to 0.25mg/L and 0.5mg/L, respectively. MIC ranges, MIC50 and MIC90 values (when applicable) of ceftazidime and ceftazidime/avibactam for the different species of Enterobacteriaceae studied are shown in Table 3. These values according to AACBL type are shown in Table 4. No significant differences were observed for the excellent ability of avibactam to protect ceftazidime from hydrolysis by different types of AACBL or species.
MIC ranges, MIC50 and MIC90 values of ceftazidime and cetazidime/avibactam displayed by the different species of AACBL-producing Enterobacteriaceae.
| MIC (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
| Ceftazidime | Ceftazidime/avibactam | ||||||
| Species | (n) | Range | MIC50 | CMI90 | Range | MIC50 | CMI90 |
| E. colia | 40 | 4 to 32 | 32 | >32 | 0.06•2 | 0.25 | 0.5 |
| K. pneumoniaeb | 29 | 8 to 32 | >32 | >32 | 0.125•1 | 0.25 | 0.5 |
| P. mirabilisc | 22 | 4 to 32 | 16 | 32 | 0.06•0.5 | 0.06 | 0.25 |
| K. oxytocad | 4 | 8 to 32 | 0.125•0.25 | ||||
| C. koserie | 3 | 4•32 | 0.125 | ||||
| E. cloacaef | 1 | >32 | 0.5 | ||||
| S. entericag | 1 | >32 | 0.5 | ||||
MIC ranges, MIC50 and MIC90 values of ceftazidime and cetazidime/avibactam for 100 AACBL-producing Enterobacteriaceae, grouped on the basis of the AACBL(s) produced. AACBL: Adquired AmpC type Beta-lactamase.
| Ceftazidime | Ceftazidime/avibactam | ||||||
|---|---|---|---|---|---|---|---|
| AACBL | (n) | Range | MIC50 | CMI90 | Range | MIC50 | CMI90 |
| CMY-type | 58 | 4 to 32 | 32 | >32 | 0.06•2 | 0.25 | 0.5 |
| DHA-1 | 35 | 8 to 32 | 32 | >32 | 0.06•1 | 0.125 | 0.5 |
| ACC-1 | 4 | 8 to 32 | 0.125•0.5 | ||||
| FOX-8 | 1 | 16 | 1 | ||||
| ACT-2 plus ACC-1 | 1 | 4 | 0.125 | ||||
| CMY-2 plus DHA-1 | 1 | >32 | 0.5 | ||||
The emergence and spread of antimicrobial resistance (ESBLs, AACBLs and carbapenemases) have become challenges for the treatment of gram-negative infections.1 This study set out to determine the activity of the novel combination agent, ceftazidime•avibactam, in well-characterized isolates of Enterobacteriaceae, including those with combined mechanisms of resistance.
Ceftazidime•avibactam is active against a wide variety of clinical ESBL-harboring Enterobacteriaceae as reported in previous studies.17 The results of the present study showed that ceftazidime•avibactam combination inhibited all ESBLs producing isolates at ≤1mg/L. The activity of ceftazidime•avibactam was not affected by the presence of more than one ESBL or any specific ESBL. Only two K. pneumoniae isolates were inhibited by higher concentrations of ceftazidime•avibactam, one a CTX-M-1 producer (MIC, 2mg/L), the other a SHV-12 producer (MIC, 8mg/L). There are no approved ceftazidime/avibactam EUCAST breakpoints,16 but after applying the FDA breakpoint, these isolates should be considered as susceptible to this combination. It is possible that other non-hydrolytic mechanisms contributing to ceftazidime resistance, such as decreased permeability or efflux pumps, were present in these isolates and also affected the activity of ceftazidime•avibactam, although further studies would be needed to elucidate the mechanism. In this respect, preliminary studies have produced no evidence of any impact of efflux on ceftazidime•avibactam activity in selected isolates of E. coli, K. pneumoniae and Enterobacter aerogenes using PAβN (phenylalanine-arginine β-naphthylamide) as efflux inhibitor during assays.18
β-Lactams penetrate the outer membrane through the porin channels and it is possible that altered expression of these porins may affect the activity of the ceftazidime•avibactam combination if it leads to reduced concentrations of the antibiotic in the periplasm. The major outer membrane porins in K. pneumoniae are OmpK35 and OmpK36.19 In order to investigate the role of these porins in susceptibility to ceftazidime•avibactam, 49 isolates with different porin profiles were selected (porin-active and porin-defective). Some of the strains presented other associated mechanisms of resistance, such as ESBLs and/or AACBLs, which made it possible to study their combined effect. Recent studies have demonstrated that OmpK35 and OmpK36 are not the major channels through which avibactam penetrates into the periplasm in K. pneumoniae18,20; these studies showed that diffusion also occurs through other pathways since the ceftazidime•avibactam MIC remained low against an OmpK35/OmpK36 mutation. The results of the present study with K. pneumoniae confirmed those results, since, against clinical isolates of ceftazidime-resistant K. pneumoniae lacking both porins, the MIC values of ceftazidime•avibactam remained lower than or equal to the FDA approved breakpoint (MIC≤8mg/L). It appears that porin deficiency does not limit the penetration of avibactam into the periplasmic space. However, the ceftazidime•avibactam MIC in these strains was higher (MIC90/MIC50 4/2) when compared to isolates expressing both porins (MIC90/MIC50 2/1). Only one isolate in the porin-active group expressing the AACBL ACT-1 showed an MIC value within the range of the porin-defective group (MIC 4mg/L). Further characterization studies would be needed for this strain. Pagèc)s et al.18 found similar results for the K. pneumoniae KP74 strain (lacking only OmpK35 and expressing the TEM-3 ESBL), which displayed a higher ceftazidime•avibactam MIC than strains lacking both porins.
Avibactam is the first β-lactamase inhibitor with activity against class C enzymes. Previous inhibitors such as clavulanic acid inhibit only class A β-lactamase enzymes. For this reason, in the present study, we tested 100 clinical isolates of ceftazidime-resistant AACBL-producing Enterobacteriaceae, including E. coli, K. pneumoniae, P. mirabilis, K. oxytoca, C. koseri, E. cloacae and S. enterica. The results showed that ceftazidime•avibactam inhibited 100% of isolates tested at ≤8mg/L, confirming the excellent ability of avibactam to protect ceftazidime from hydrolysis by different types of AACBL in different species, as Karlowsky et al. also showed.17 Moreover, no significant differences were found for ceftazidime•avibactam MIC values associated with any specific AACBL.
The results of the present study demonstrate the potent activity of avibactam to hydrolyze ESBLs and AmpC in Enterobacteriaceae. These data combined with the results reported by Lahiri et al.21 demonstrate that the development of high-level resistance to ceftazidime/avibactam appears to occur at a low frequency and provides evidence that ceftazidime•avibactam is an excellent alternative for the treatment of infections caused by multidrug-resistant isolates.
Nevertheless, the ceftazidime•avibactam MIC of 8mg/L against a K. pneumoniae isolate producing SHV-12 obtained in the present study, which is just within the limit of the currently accepted FDA breakpoint, gives cause for concern.
The results of this study will have to be evaluated in the future when EUCAST validates a susceptibility breakpoint for ceftazidime•avibactam. In the case that the breakpoint equaled the current EUCAST breakpoint for ceftazidime alone (susceptible, ≤1mg/L),16 the susceptibility category of some isolates examined in this study would n to be modified.
In conclusion, the results of this study, using a wide range of isolates and well characterized resistance mechanisms, support previous data and confirm the potential utility of ceftazidime•avibactam as a therapeutic agent for treating serious infections caused by multidrug-resistant Enterobacteriaceae.
Financial supportThis study was partially supported by a grant from Astra-Zeneca Laboratories.
Conflicts of interestL. Martínez-Martínez has been a speaker for Pfizer, Merck, Janssen-Cilag and AstraZeneca, he has been scientific advisor for Pfizer, and he has received research grants from Pfizer, Merck, Janssen-Cilag and AstraZeneca.
This work was partially supported by the Plan Nacional de I+D+i and the Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015) • co-financed by European Development Regional Fund “A way to achieve Europe” ERDF.
Part of this study was presented at the European Congress of the Society of Microbiology and Infectious Diseases (26th ECSMID) held in Amsterdam from 9 to 12 April 2016.