The routine use of a single aerobic bottle for blood culture in pediatric patients has become commonplace, as anaerobic bacteria are not frequently involved in clinically significant infections. The aim of this study was to assess the usefulness of routinely performing anaerobic blood cultures in pediatric oncology patients.
MethodsProspective study was conducted on pediatric (<18 years) patients affected with febrile syndrome after receiving chemotherapy for hematological or solid malignancies. Samples were inoculated into pediatric aerobic and standard anaerobic bottles (BacT/Alert automatic system). Strains were considered clinically significant, or deemed as contaminants, depending on isolation circumstances and clinical criteria.
ResultsA total of 876 blood cultures from 228 patients were processed during the 21-month study period (January 2014 to September 2015). Baseline diagnosis included 143 solid tumors and 67/18 cases of leukemia/lymphoma. Bacterial growth was detected in 90 (10.2%) blood cultures for 95 different isolates, of which 62 (7.1%)/63 isolates were considered clinically significant. Among the latter, 38 (60.3%) microorganisms grew in both aerobic and anaerobic bottles, 18 (28.6%) only in aerobic bottles, and 7 (11.1%) only in anaerobic bottles. Gram-negative bacilli (33; 52.4%), mainly from the Enterobacteriaceae family, were the most frequently isolated microorganisms. Overall, only 3 out of 90 isolates (3.3%) were strict anaerobes (Propionibacterium acnes), and all of them were deemed contaminants.
ConclusionStrict anaerobes did not cause significant infections in febrile pediatric oncology patients, and anaerobic blood culture bottles offered no additional advantages over aerobic media. Our results suggest that routine blood cultures should be solely processed in aerobic media in this group of patients.
En pacientes pediátricos es habitual el procesamiento de hemocultivos únicamente en medio aerobio, debido a la escasa relevancia de los microorganismos anaerobios en la etiología infecciosa habitual. El objetivo de este estudio es valorar la utilidad del uso rutinario del medio de cultivo anaerobio en pacientes oncológicos pediátricos.
MétodosEstudio prospectivo en pacientes pediátricos (<18años) en tratamiento quimioterápico de procesos oncológicos con síndrome febril. Las muestras se inocularon en botellas aerobias pediátricas y anaerobias estándar (sistema automático BacT/Alert). Las cepas aisladas fueron consideradas clínicamente significativas o contaminantes en función de las circunstancias de aislamiento y la clínica del paciente.
ResultadosDurante el periodo de estudio (enero 2015-septiembre 2016) se procesaron 876 hemocultivos procedentes de 228 pacientes diagnosticados de tumores sólidos (143) y leucemia/linfoma (67/18). Se detectó crecimiento en 90 (10,2%) hemocultivos y se aislaron 95 cepas, de los cuales 62 (7,1%), correspondientes a 63 cepas, se consideraron clínicamente significativos. Entre estos últimos, 38 (60,3%) microorganismos crecieron en ambas botellas, 18 (28,6%) únicamente en aerobiosis y 7 (11,1%) únicamente en anaerobiosis. Bacilos gram negativos (33; 52,4%), mayoritariamente enterobacterias, fueron los más frecuentemente aislados. Solo 3 (3,3%) de los microorganismos aislados eran anaerobios estrictos (Propionibacterium acnes), y todos ellos fueron considerados contaminantes.
ConclusiónMicroorganismos anaerobios están raramente involucrados en infecciones en pacientes oncológicos pediátricos, y la utilización de botellas anaerobias no ofrece ninguna ventaja adicional. Según nuestros resultados es suficiente el uso de medio aerobio en el procesamiento de los hemocultivos en este tipo de pacientes.
Infections are a frequent complication in patients with oncologic and hematologic malignancies, bloodstream infections (BSI) being one of the most common and severe, mainly accounted for chemotherapy-induced neutropenia alongside invasive procedures. Standard procedures for processing blood cultures (BC) in adult patients include both aerobic and anaerobic bottles, but in pediatric patients, anaerobic bacteria are not frequently involved, particularly in BSI.1–3 Nowadays, in pediatric patients the routine use of a single aerobic bottle for BC has become commonplace.
In our hospital, according to our protocol, blood samples from pediatric patients are inoculated only in pediatric aerobic bottles, except in selected patients presenting with clinical conditions that increase the risk of anaerobic infection, such as neutropenia, corticosteroid therapy or central venous catheters.1–3
Although chemotherapy and treatment procedures for malignant diseases have evolved swiftly in recent years, updated information on anaerobic etiology of BSI in this specific group of pediatric patients is lacking. The aim of this study was to assess the current etiology of BSI in our geographical area and the usefulness of routinely performing anaerobic BC in pediatric oncology patients presenting with fever, usually in the setting of chemotherapy-induced neutropenia (FN).
Material and methodsWe present an observational prospective study in a cohort of oncology pediatric patients (<18 years at inclusion) that present with fever during chemotherapy, in most cases during neutropenia periods, in Hospital Sant Joan de Déu (Barcelona), a tertiary-care pediatric-maternal university hospital with 350 beds that serves a geographical area containing approximately two million people; our center also receives patients from the rest of Catalonia and Spain who require treatment for severe illnesses. The local ethics committee approved the study.
Blood samples were routinely taken by trained officers, splitted in two equal volumes and inoculated into one pediatric aerobic BacT/Alert PF bottle and into one standard anaerobic BacT/Alert SN bottle, to be later processed using BacT/Alert (BioMérieux, Durham, NC, USA) automatic incubation system. Our BC collection protocol recommends an optimal blood sample of at least 4mL per bottle. In this study, due to the characteristics of the patients, the final volume was usually only between 1 and 2mL per bottle, although any volume available was accepted. Most samples were obtained from intravascular devices (IVD), usually a one-lumen tunneled central venous catheter (Port-A-Cath). BC were performed at onset of each FN episode, and again after 48–72h if fever persisted despite antibiotics. Isolates were considered clinically significant if one of the following criteria were met: (a) when the isolated microorganism was considered a usual pathogen (e.g. enteric gram-negative rods); (b) when a microorganism usually considered contaminant (e.g. coagulase-negative staphylococci) was isolated in both aerobic and anaerobic bottles during the first 48h of incubation; (c) when the same usually contaminant strain was persistently isolated in consecutive BC; or (d) was IVD-related: BSI was judged to be IVD-related when fever coincided with the use of the device, when the same strain was isolated from BC and the device exit site, when resolution of symptoms coincided with the removal of the IVD or when the same microorganism was isolated from the device culture after its removal.
In order to establish the clinical significance of each isolate, clinical and microbiological data were collected and assessed prospectively together with the physician in charge of the patient. Dubious cases under full antibiotic treatment were considered clinically significant in this study.
Statistical analysisCategorical variables were described as percentages, and continuous variables as mean/standard deviation (SD) or median/ranges.
ResultsDuring the 21-month study period (January 2014–September 2015), 876 BC (aerobic and anaerobic paired bottles) from 228 patients (43.4% females, mean age: 6.7 years (SD: 4.9), ranging from 6 months to 18 years) were processed in the microbiology laboratory. Baseline diagnosis included solid tumors n=143 (affecting central nervous system, n=62; muscular and skeletal, n=28; kidney n=12; histiocytosis n=10; and others n=31), acute leukemia (n=67) and lymphoma (n=18). Among patients with solid tumors, there were 22 patients that had received an autologous peripheral blood stem cell transplantation. No patient included in this study underwent an allogeneic stem cell transplantation.
Bacterial growth, yielding 95 isolates, was detected in 90 BC (10.2%) of which 62 (7.1%), corresponding to 63 isolates, were considered clinically significant and 28 (3.2%), corresponding to 32 isolates (17 coagulase-negative staphylococci, 5 viridians streptococci group, 5 Micrococcus spp., 1 Corynebacterium spp., 1 Moraxella spp. and 3 Propionibacterium acnes), were deemed contaminants. In 5 BC two different microorganisms were simultaneously isolated, of which only one case (Escherichia coli and Klebsiella pneumoniae) was considered true bacteremia.
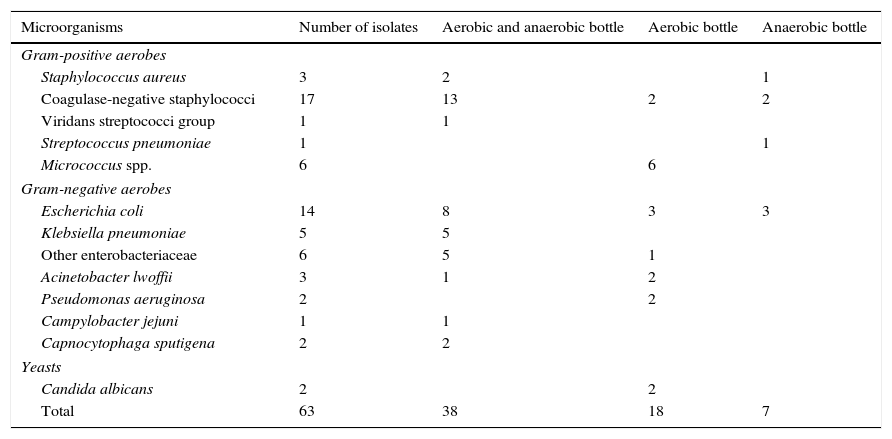
Taking into account only the 63 clinically significant microorganisms (Table 1), 38 (60.3%) of them grew in both aerobic and anaerobic bottles, 18 (28.6%) only in aerobic bottles and 7 (11.1%) (1 Staphylococcus aureus, 2 coagulase-negative staphylococci, 1 Streptococcus pneumoniae and 3 E. coli) only in anaerobic bottles. Altogether, 56 clinically significant isolates (88.9%) grew in aerobic bottles and 45 (71.4%) in anaerobic bottles.
Clinically significant microorganisms isolated from blood cultures of pediatric oncology patients and the type of bottle from which they were recovered.
| Microorganisms | Number of isolates | Aerobic and anaerobic bottle | Aerobic bottle | Anaerobic bottle |
|---|---|---|---|---|
| Gram-positive aerobes | ||||
| Staphylococcus aureus | 3 | 2 | 1 | |
| Coagulase-negative staphylococci | 17 | 13 | 2 | 2 |
| Viridans streptococci group | 1 | 1 | ||
| Streptococcus pneumoniae | 1 | 1 | ||
| Micrococcus spp. | 6 | 6 | ||
| Gram-negative aerobes | ||||
| Escherichia coli | 14 | 8 | 3 | 3 |
| Klebsiella pneumoniae | 5 | 5 | ||
| Other enterobacteriaceae | 6 | 5 | 1 | |
| Acinetobacter lwoffii | 3 | 1 | 2 | |
| Pseudomonas aeruginosa | 2 | 2 | ||
| Campylobacter jejuni | 1 | 1 | ||
| Capnocytophaga sputigena | 2 | 2 | ||
| Yeasts | ||||
| Candida albicans | 2 | 2 | ||
| Total | 63 | 38 | 18 | 7 |
Apart from strict aerobes (n=13, 20.6%: Pseudomonas aeruginosa, Acinetobacter lwoffii, Micrococcus spp. and Candida albicans) that usually grow only in aerobic bottles, the rest of clinically significant isolates were facultative aerobic/anaerobic microorganisms of which 37 (74%) grew in both aerobic and anaerobic bottles, 6 (12%) only in aerobic bottles and 7 (14%) only in anaerobic bottles.
Among clinically significant isolates, gram-negative rods (33; 52.4%), mainly belonging to the Enterobacteriaceae family (n=25; 75.8%), were the more frequently isolated microorganisms, followed by gram-positive cocci (n=28; 44.4%), mainly coagulase-negative staphylococci (n=17; 60.7%). One episode of infection (2 isolates) accounted for yeasts. Overall, only 3 isolates were strict anaerobes (Propionibacterium acnes) and all of them were deemed contaminants.
In 5 patients the same strain (1 coagulase-negative staphylococci, 2 Micrococcus spp., 1 Acinetobacter lwoffii and 1 Candida albicans) was isolated in two different BC during the same episode of infection.
In 6 patients the same strain (Escherichia coli, Micrococcus spp. and coagulase-negative Staphylococcus, 2 patients each) was isolated in two or more episodes (one strain of Escherichia coli was isolated in three episodes), and the infection was considered to be related with persistent IVD colonization.
Overall, after discarding contaminant microorganisms and taking into account only one isolate of the same strain in each episode of infection, 57 episodes, attributable to 58 microorganisms, of BSI in 50 FN pediatric patients were microbiologically confirmed.
DiscussionOur results show a predominance of isolates belonging to Enterobacteriaceae family and Staphylococcus spp. in pediatric patients with FN, which is in accordance with previous studies, carried out in both pediatric4,5 and adult6,7 patients, and also with our own previous experience,8 when anaerobic BC were routinely performed in our hospital. These results confirm that strictly anaerobic bacteria are very seldom involved in oncology pediatric patients with FN; actually, we did not detect a single episode of BSI due to these microorganisms in our large prospective study. Consequently, neutropenia did not enhance the risk of anaerobic BSI as compared with general pediatric patients in our hospital.8 Therefore, if the only goal of anaerobic bottles were the isolation of strict anaerobes, it would not be worth to use them. Nevertheless, the influence of systematic anaerobic BC bottles in the isolation of facultative aerobic/anaerobic and strict aerobic microorganisms needs to be considered.
With regard to strict aerobic microorganisms, the isolation of yeasts in BC from patients with FN in our center has progressively decreased in recent years and at present its incidence rate is very low, probably due to antifungal prophylactic treatments. Nevertheless non-fermenting gram-negative rods remain frequently isolated. In our study, strict aerobic microorganisms (including those Micrococcus spp. that were considered clinically significant) accounted for 15.5% of BSI episodes. It is important to point out that many of these microorganisms are clinically less predictable, display greater variability in terms of resistance to antibiotics than anaerobic pathogens,9–11 and may not be covered by standard empirical antibiotic treatment; others, like yeasts, need specific treatment. Therefore, their proper microbiological diagnosis is of greater clinical importance.
We observed that when facultative aerobic/anaerobic microorganisms were detected only in one bottle, there were no differences between aerobic and anaerobic bottles (12% and 14% respectively). These data suggest that anaerobic BC offer no diagnostic advantage for this group of bacteria in children with FN.
Why facultative aerobic/anaerobic bacteria grew only in one bottle would be explained by a random event, more likely related with low bacterial concentration in the specimen and small sample volume than with the aerobic or anaerobic atmosphere itself.1 It is important to point out that the greater the volume of blood inoculated, the greater the yield from blood cultures.12 This is particularly relevant in pediatrics because of the difficulty of getting an optimal volume of blood sample in some patients.
Moreover, because a specific pediatric anaerobic bottle is not commercially available, the volume of media in each anaerobic bottle was twice the volume in each pediatric aerobic bottle, but the volume of inoculated blood was the same. This diminishes the concentration of bacteria by one half in the culture medium and might contribute to lower the rate of isolation in anaerobic BC. Therefore, it is likely that bacterial recovery would potentially be enhanced by inoculating the entire sample into the aerobic medium.
An important issue, in practical terms, is how to discriminate contamination from infection when common saprophytic bacteria are involved (coagulase-negative staphylococci, Micrococcus spp., Corynebacterium spp., Streptococcus viridans, Propionibacterium spp., Bacillus spp., etc.).13 In our study these microorganisms were considered etiologically related with 36.2% of BSI episodes. Usually, in pediatric oncology patients, fever is the most common and earliest symptom of BSI. But fever may also occur due to the administration of blood products or as an adverse event of cytotoxic therapy. In addition, almost all patients carry permanent IVD during the treatment period, which are known to get contaminated by these microorganisms, often leading to BSI.14 There are not specific and definitive microbiological criteria to discriminate between contamination and BSI, but the isolation of these microorganisms from at least two blood samples would back up their etiological role.1,14
In conclusion, considering that strict anaerobes rarely cause BSI in pediatric patients with malignancies and FN and also the difficulty to obtain blood samples of sufficient volume to ensure an optimal diagnostic approach in these patients, we think, consistently with other authors’ opinions,11,15 that BC should be solely processed in aerobic medium in these patients, in order to enhance the isolation of the most common microorganisms, especially strict aerobes.
FundingThis article has not received specific funding.
Conflict of interestThe authors of this article have no conflict of interest in relation to its content.