We present the case of a 62-year-old woman diagnosed with a haematological disease, monoclonal gammopathy, in 2006, which developed into quiescent multiple myeloma in 2009 and symptomatic myeloma (IgG kappa, ISS 1) in 2011. She underwent autologous transplant of peripheral blood haematopoietic progenitors (AHSCT), after which she suffered a relapse in October 2014, with a discreetly hypocellular bone marrow, with 7% intermediate maturation plasma cells. After receiving 10 sessions of radiation therapy and 4 cycles of VTD (bortezomib+thalidomide+dexamethasone), the patient was admitted to the Haematology Department of the Hospital Universitario Central de Asturias in April 2015 to receive an allogeneic transplant of haematopoietic precursors from a HLA-identical sibling, conditioned with fludarabine. Furthermore, at the time of admission, she had a persistent catarrhal clinical picture with cough, without expectoration, fever, or dyspnoea, so she was given levofloxacin (500mg IV/24h).
The procedure was performed without incident and prophylaxis for graft-versus-host disease was initiated with methotrexate and cyclosporin. In the history recorded on the following days, the patient reported oral pain, with a WHO grade 3 mucositis being detected that required parenteral nutrition. On the sixth day post-transplant, she started with a fever of 39°C, and the blood work showed a deep medullar aplasia (leukocytes 0.00×103/μl, red blood cells 2.76×106/μL, haemoglobin 8.7g/dl, platelets 13,000/μL), with liver and kidney function tests showing values within normal limits. Blood cultures were taken (BD BACTEC® Plus Aerobic/Anaerobic F), and treatment with piperacillin-tazobactam was started (4g IV/6h), according to the hospital's antibiotic therapeutic protocol for febrile neutropenia.
At 32h, the anaerobic bottles were positive, while the aerobic bottles were positive at 57h, gram-negative bacilli with a spindle-shaped appearance were observed in staining. In subcultures in a Brucella, chocolate blood agar some greyish colonies with dry appearance grew at 18h. Identification was performed using MALDI-TOF, obtaining a score of 2.1 for Leptotrichia trevisanii. This result was confirmed through 16S rRNA sequencing, comparing the sequence obtained with the GenBank® database and using the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
The antibiotic sensitivity was performed by broth microdilution, according to the reference method for anaerobic micro-organisms and the breakpoints indicated in the Clinical and Laboratory Standards Institute (CLSI). The micro-organism was sensitive to the following antibiotics: penicillin (MIC=0.12mg/L), amoxicillin (MIC=1mg/L), piperacillin (MIC≤16mg/L), amoxicillin-clavulanic (MIC=0.5/0.25mg/L), piperacillin-tazobactam (MIC=16/4mg/L), cefoxitin (MIC=1mg/L), imipenem (MIC=0.12mg/L), chloramphenicol (MIC=8mg/L), clindamycin (MIC≤0.5mg/L), tetracycline (MIC≤2mg/L) and metronidazole (MIC≤0.5mg/L). Moxifloxacin (MIC=8mg/L) was classed as resistant. There are no breakpoints in the CLSI for erythromycin, but the MIC obtained (64mg/L) was high.
The first case of bacteremia by Leptotrichia trevisanii was described by Tee et al. in a male with acute myeloid leukaemia in 2001,1 and at least 12 cases have been published since then.2–8 The patients involved suffered from some type of haematologic disease, except one, who had oesophageal cancer with metastases in the liver, lung and lymph nodes,2 which shows the opportunistic nature of this micro-organism. All of them described a situation of febrile neutropenia as a predisposing factor of sepsis, accompanied by the appearance of lesions in the oropharyngeal mucosa. These constitute a route for bacterial translocation,2,3 which can trigger bacteremia in situations of medullar aplasia.
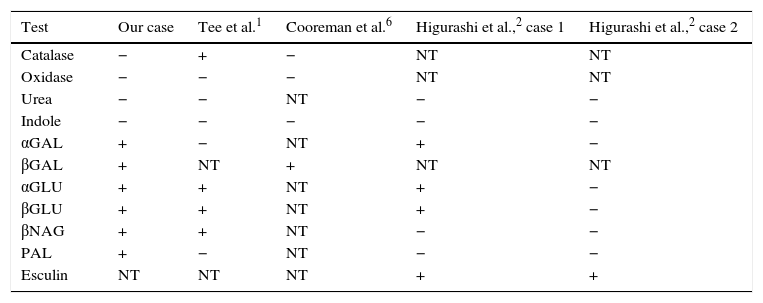
Phenotypic batteries are not able to identify Leptotrichia spp., since it presents a low biochemical reactivity.3,4 In addition, the comparison between the different strains described demonstrates the difficulty in identifying the micro-organism based solely on biochemical tests, due to the variability of the results (Table 1). In our case, the API® Rapid/ID 32 A (bioMérieux®) system was used. The result of the number profile obtained (0411400000) was Clostridium acetobutylicum (87.2%), which cannot be correlated with the gram-negative spindle bacilli observed under the microscope.
Comparison of the results of biochemical tests between different strains of Leptotrichia trevisanii.
| Test | Our case | Tee et al.1 | Cooreman et al.6 | Higurashi et al.,2 case 1 | Higurashi et al.,2 case 2 |
|---|---|---|---|---|---|
| Catalase | − | + | − | NT | NT |
| Oxidase | − | − | − | NT | NT |
| Urea | − | − | NT | − | − |
| Indole | − | − | − | − | − |
| αGAL | + | − | NT | + | − |
| βGAL | + | NT | + | NT | NT |
| αGLU | + | + | NT | + | − |
| βGLU | + | + | NT | + | − |
| βNAG | + | + | NT | − | − |
| PAL | + | − | NT | − | − |
| Esculin | NT | NT | NT | + | + |
+: positive; − negative; NT: not tested.
The MALDI-TOF mass spectrometry has proved to be a cost-effective and rapid tool in the final identification of micro-organisms for which conventional methods are inconclusive. Martín-Gutiérrez et al. managed to correctly identify the Leptotrichia trevisanii species just 2h after the blood culture was positive,3 the same as Schmitt et al., who did so in a period of 48h.5 In our case, it allowed us to establish the aetiology of the bacteremia within 18h after the passage of positive blood cultures to solid culture media.
The sensitivity patterns for Leptotrichia spp. are not defined, although it has been described as sensitive to most antimicrobials.1,2,6,7 There is not enough experience to establish a treatment of choice. In the reviewed literature several therapeutic regimens were implemented based on the sensitivity study, with resolution of the clinical picture in all cases. Our patient was treated with piperacillin-tazobactam, the blood cultures being negative on the tenth day after treatment and with resolution of mucositis on day 15. However, Martín-Gutiérrez et al. did not observe clinical improvement using piperacillin-tazobactam, changing the treatment to meropenem.3
The use of levofloxacin is inadequate in these cases, since Leptotrichia trevisanii shows in vitro resistance to fluoroquinolones.2,6 Schrimsher et al. described 3 clinical cases in which levofloxacin was used as an empirical treatment in febrile neutropenia, which did not prevent the development of bacteremia by Leptotrichia.8 Therefore, it is important that patients with febrile neutropenia and mucositis, more prone to infections by anaerobic micro-organisms, are treated with anaerobicidal antimicrobials.7,8
Please cite this article as: Sabater Cabrera C, Fernández Blázquez A, García Carús E. Bacteriemia por Leptotrichia trevisanii en una paciente sometida a trasplante alogénico de médula ósea. Enferm Infecc Microbiol Clin. 2017;35:389–390.