We present the case of a 70-year-old Caucasian man, living in a rural area of the province of Madrid with no known toxic habits. Among his personal history, the following is of note: type 2 diabetes mellitus and well-differentiated, stage IV adenocarcinoma of the rectum (pT3 N0 M1 due to bilateral pulmonary metastases, according to the 2017 TNM classification), undergoing active chemotherapy treatment for systemic disease (second line), based on 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX) and cetuximab, of which he has completed five cycles with good tolerance.
He consulted due to a brownish, painless macule with erythematous borders on the front side of his right leg, measuring approximately 2cm×2cm in diameter, which had appeared 72h previously. In the systems review, he denied having a fever, the onset of skin lesions in other places, loco-regional trauma, insect bites and other compatible inflammatory/infectious symptoms. Similarly, there were no data suggestive of immunosuppression in the laboratory tests. The patient had never left Spain.
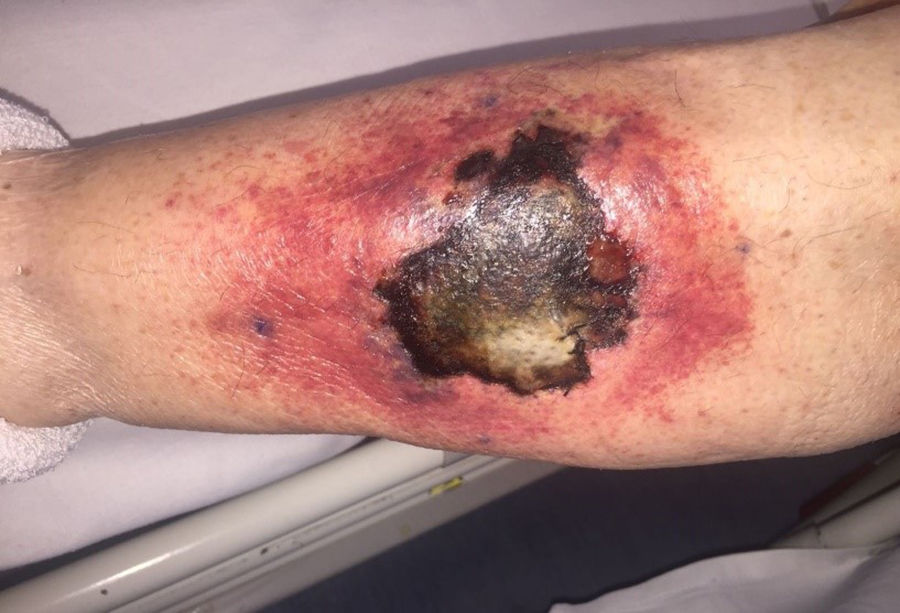
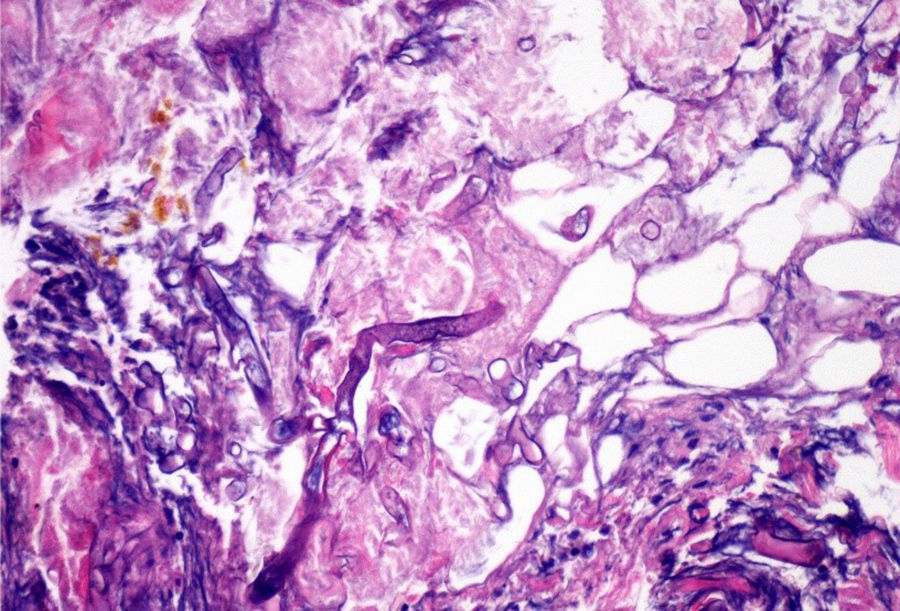
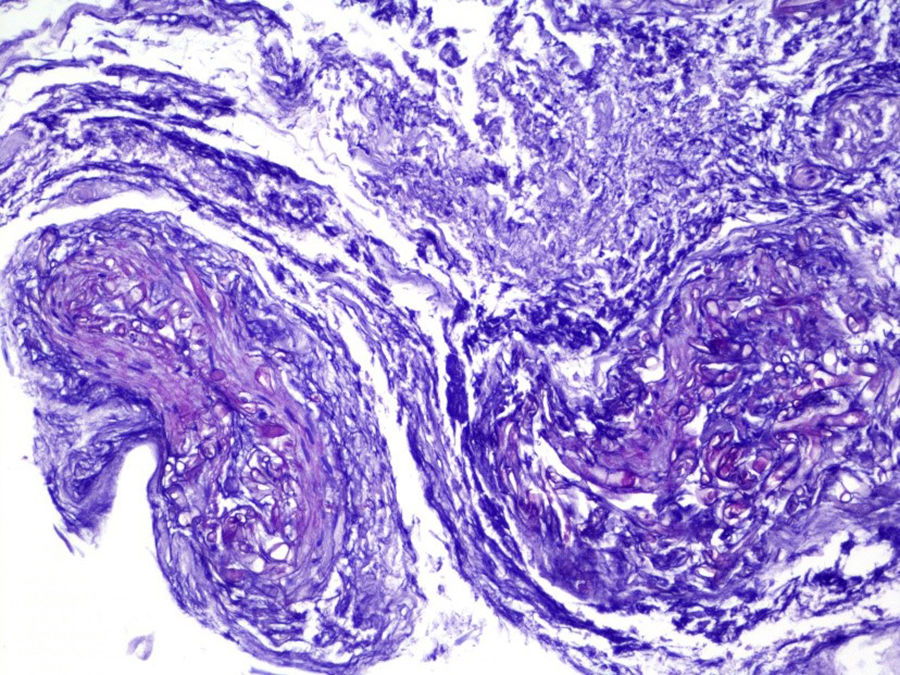
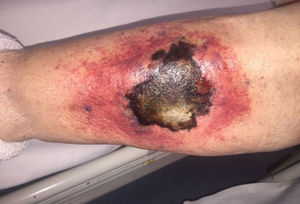
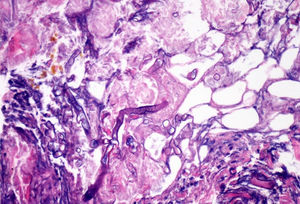
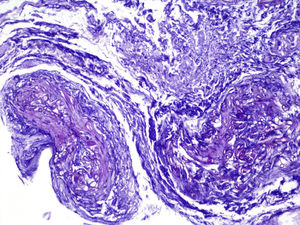
ProgressIn the first 48h after admission, the lesion developed, with spreading of the erythematous borders, which also turned violet, and the central macule, which became ulcerous with a necrotic background (Fig. 1) and spontaneously painful. Samples were collected for culturing and a skin biopsy was taken, the analysis of which showed the presence of necrotic and ischaemic lesions, as well as the existence of fungi with large, non-partitioned hyphae in the dermis and hypodermis, some of which passed into the blood vessels (Figs. 2 and 3). In the culture taken from the ulcer, a strain of Rhizopus oryzae eventually grew.
The differential diagnosis of skin ulcers caused by fungi must include the following genera: Candida, Aspergillus, Cryptococcus, Fusarium, Trichosporon, Paecilomyces and the group of zygomycetes. In the case report presented (black skin ulcer) and with presumed fungi forming non-partitioned hyphae, the most likely diagnosis is a genus belonging to the order Mucorales, the infection of which is more commonly known as mucormycosis. In the division of zygomycetes, two orders of fungi can be found: Entomophthorales (entomophthoromycosis) and Mucorales (mucormycosis). The genera Basidiobolus and Conidiobolus belong to the order Entomophthorales, parasitic fungi of some insects. Infections in humans tend to be subcutaneous chronic granulomatous diseases and they have been documented mainly in Africa, South America, India and the Middle East. Within the order Mucorales, the larger and more extensively-studied order, there are 12 families, of which the main family causing disease in humans is the Mucoraceae. The genera included in the Mucoraceae family are Rhizopus, Mucor, Rhizomucor, Lichtheimia and Apophysomyces. Other genera capable of causing disease in humans are Saksenaea, Cunninghamella and Syncephalastrum.1 All of these are distributed throughout nature, being found mainly in decaying vegetation and in soil. Some species included in this group may be identified with the naked eye as mould-formers, for example, on rotting bread or potatoes.
Arnold Paltauf was the first physician to histologically identify a specimen causing mucormycosis, specifically Mycosis mucorina (University of Graz, Austria), in 1885.
It is estimated that the incidence of this type of infection in countries with validated registries is approximately 1–1.5cases/million/year.2 These fungi are known to be fast-growing (they may produce macroscopically visible woolly colonies in 10–15h) and to reproduce from asexual spores (sporangiospores) which may be transported through the air. Human beings are in contact with these structures on a daily basis, given their ubiquitous distribution, although we do not develop infection thanks to the efficiency of the immune system. Mucormycosis is therefore considered an infection which is typical of immunosuppressed patients, with the main predisposing factors being diabetes (in particular in case of ketoacidosis), haematological malignancies, haematopoietic stem cell transplantation or solid organ transplantation, prolonged treatment with glucocorticoids, treatment with deferoxamine, iron overload, and contact of skin and mucosa with contaminated medical equipment (adhesive bandages, hospital bedding, tongue depressors, etc.).
The infection may be acquired through inhalation, ingestion or contamination of wounds by the spores present in the medium.2,3In vivo, zygomycetes grow forming flat hyphae without or with very limited non-pigmented walls. The hyphae are usually large (>10μm) and irregular or amorphous. Another characteristic which is typical of the Mucorales, is their capacity to invade blood vessels, with the infarction of infected tissues (macroscopic black eschar) being a distinctive feature.
The clinical forms of human mucormycosis include2,4:
- –
Rhino-orbital-cerebral: the most common form. Acquired from the inhalation of spores, with the paranasal sinuses being the first anatomical structures affected. The infection progresses with headache, facial pain, fever and purulent nasal secretions. Without treatment, it tends to progress quickly, subsequently involving the palate, nasal passages and bony orbit.
- –
Pulmonary: acquired from the inhalation of spores, which are deposited in the bronchioles and alveoli. The clinical picture is one of necrotising pneumonia, which may develop with fever and life-threatening haemoptysis. Without treatment, it may spread towards the mediastinum or other organs via the haematogenous route.
- –
Gastrointestinal: rare. Tends to occur after the intake of foods contaminated with spores. The stomach is the most commonly affected site. It may develop with abdominal pain and haematemesis. Macroscopically, necrotic lesions and ulcers are observed, which may progress to perforation and peritonitis.
- –
Cutaneous: result of the inoculation of spores into the dermis. An epidemiological history of trauma or burns is common.
- –
Disseminated disease: very rare, being present at times of severe immunosuppression, in major burns or patients who have received high doses of deferoxamine. Its mortality rate is close to 100%.
Any clinical form can be diagnosed quickly through the identification of non-partitioned and broad hyphae in the tissue sample. This is possible with the direct examination of material treated with KOH and calcofluor white. Histopathological sections can be stained with haematoxylin–eosin (H&E) or periodic-acid Schiff (PAS). Cultures may not produce growth in up to 50% of cases. 1,3-β-d-Glucan and the galactomannan antigen are not useful as diagnostic tests in mucormycosis, since these fungi do not show said components in their cell wall.2,4
The treatment of mucormycosis should be based on two pillars: the removal of the fungal material from the infected tissues and reconstitution of the immune system (or the underlying disease) of the patient. The combination of surgery with prolonged anti-fungal treatment is mandatory, whatever the patient's clinical picture.2 Surgery should include broad and intensive debridement of the affected tissues, as soon as the diagnosis is suspected.
The initiation of antifungal medicines is empirical, with liposomal amphotericin B (dose 5mg/kg/day) being the drug of choice to start with.2,4 Subsequently, provided the patient has an adequate clinical response, this may be changed to oral posaconazole or isavuconazole (step therapy). The standard dose of posaconazole is 300mg/12h on the first day, followed by 300mg/24h from the following day. In the case of isavuconazole, the loading dose lasts 48h (200mg/8h), with it subsequently being reduced to 200mg/24h.
In cases with a limited response or intolerance to amphotericin B, a rescue therapy based on replacing amphotericin with posaconazole or isavuconazole may be used. However, the intravenous route should be used initially in these patients, and maintained until a favourable clinical response is obtained. The loading doses are the same as those for the oral route.
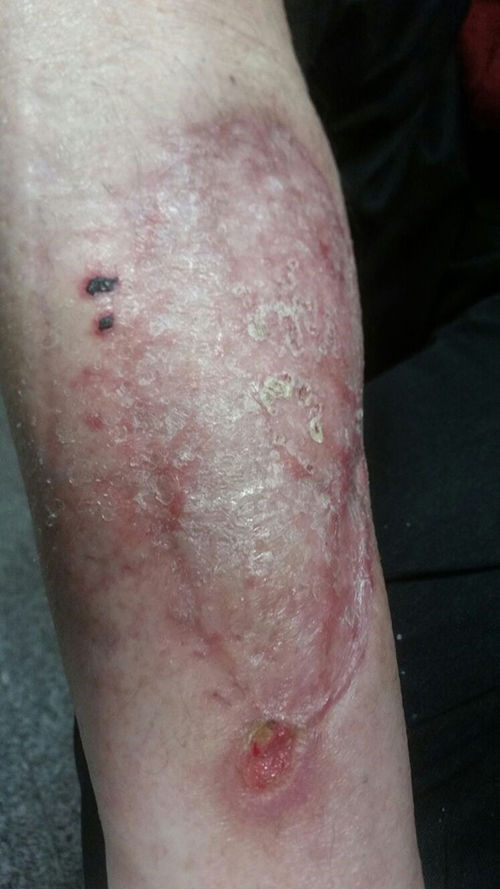
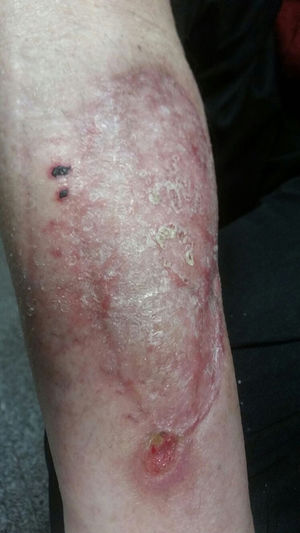
There is no standardised duration of antifungal treatment. The use of liposomal amphotericin B should be maintained until a favourable clinical response is achieved, which tends to take several weeks, or until intolerance or clinical worsening occurs. Subsequently, the use of azoles should be prolonged until the signs and symptoms of infection have completely resolved, which may last for several months. This situation is more common in patients with prolonged immunosuppression. In the case presented, surgical debridement and margin extension was carried out until unaffected tissue was found. Subsequently, after checking that the wound (which was left uncovered) had progressed favourably, reconstruction was carried out with a skin graft. From the outset, the patient received liposomal amphotericin B (5mg/kg/day) for a total of 14 days. Subsequently, he was discharged on oral posaconazole (300mg/day), a treatment that continued for eight weeks. The final clinical outcome is shown in Fig. 4.
Please cite this article as: Soto Castillo JJ, Fortún Abete J, Soria-Rivas A. Úlcera negra en pierna. Enferm Infecc Microbiol Clin. 2019;37:344–346.