We present the case of a 64-year-old man with a history of stress incontinence, long-term hypercholesterolaemia and controlled non-insulin-dependent diabetes mellitus. He underwent a radical retropubic prostatectomy in 2013 due to a stage 1 prostate carcinoma and received post-surgical treatment with surgical bed radiotherapy in 2014 due to recurrence of the prostate cancer. He was admitted for a stress incontinence test with increased left hemi-scrotum volume. This was diagnosed as a 230ml left-sided hydrocele by means of ultrasonography.
The patient underwent surgery and a hydrocelectomy was performed with an incision in the left hemi-scrotum and dissection of the layers of the scrotum up to the tunica vaginalis. A thick, purulent and non-foul smelling substance was obtained at the opening of the tunica vaginalis. It was squeezed out and a sample was taken for Microbiology. The walls of the cyst were dried out and a drain left in place. Empirical treatment with amoxicillin/clavulanic acid (500/125mg/8h) was started for 10 days, with favourable progress.
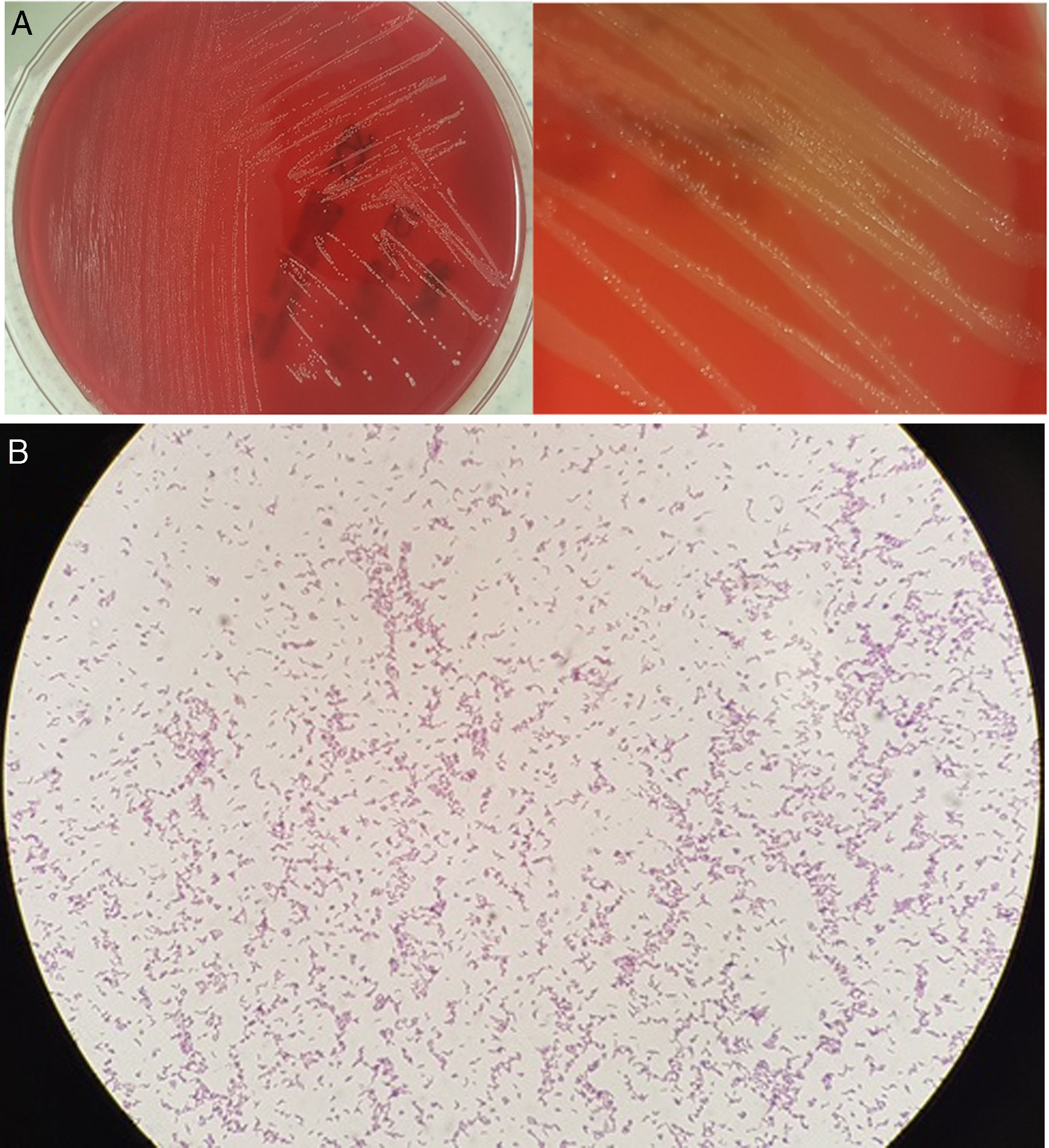
Microbiological diagnosisA surgical sample of the hydrocele contents was aspirated using a syringe. It was seeded in blood agar, chocolate agar and thioglycollate broth, with these media incubated in aerobic conditions (37°C), and in Wilkins–Chalgren agar supplemented with 5% sheep blood agar in anaerobic conditions (37°C). After 48h of incubation in anaerobic conditions, faint growth of minute colonies was observed. After 72h, the growth was visible with discreetly beta-haemolytic colonies of a macroscopic morphology similar to that of Streptococcus (Fig. 1A). In the Gram stain, gram-positive bacilli with a dimorphic and slightly curved morphology were observed (Fig. 1B). These colonies were identified by means of mass spectrometry (MALDI-TOF®, Bruker-Daltonics) as Actinotignum urinale. On the plates incubated in aerobic conditions, faint growth was obtained after the third day of incubation. Antibiotic susceptibility testing was performed using concentration gradient diffusion strips (ETEST®, bioMérieux) in anaerobic conditions, with the following minimum inhibitory concentrations (mg/l): penicillin<0.016, amoxicillin/clavulanic acid 0.016, piperacillin/tazobactam<0.016, imipenem<0.002, clindamycin 0.016, interpreted as susceptible, and metronidazole>256 as resistant. This was in accordance with the 2017 EUCAST criteria, version 7.0, for anaerobic gram-positive bacilli, since there are no established criteria for these microorganisms. No other patient samples were examined for the microbiological study.
The strain was sent to the Taxonomy Laboratory of the Spanish National Microbiology Centre, which forms part of the Instituto de Salud Carlos III, to check the identification by means of 16S rRNA gene sequencing, where the genus and species were confirmed.
Final commentsTreatment of a non-infected hydrocele is always surgical. Infected hydroceles, however, are usually a surgical finding and their infectious aetiology may be reactive to a contagious genitourinary and anorectal infection. The microorganisms involved may be responsible for sexually transmitted diseases, gram-positive cocci, Enterobacteriaceae and anaerobes in cases of gangrene. Viruses (varicella-zoster), Brucella, filariasis and bacillus Calmette-Guérin are less common in case of bladder cancer treatment. Finally, emerging pathogens such as A. urinale.
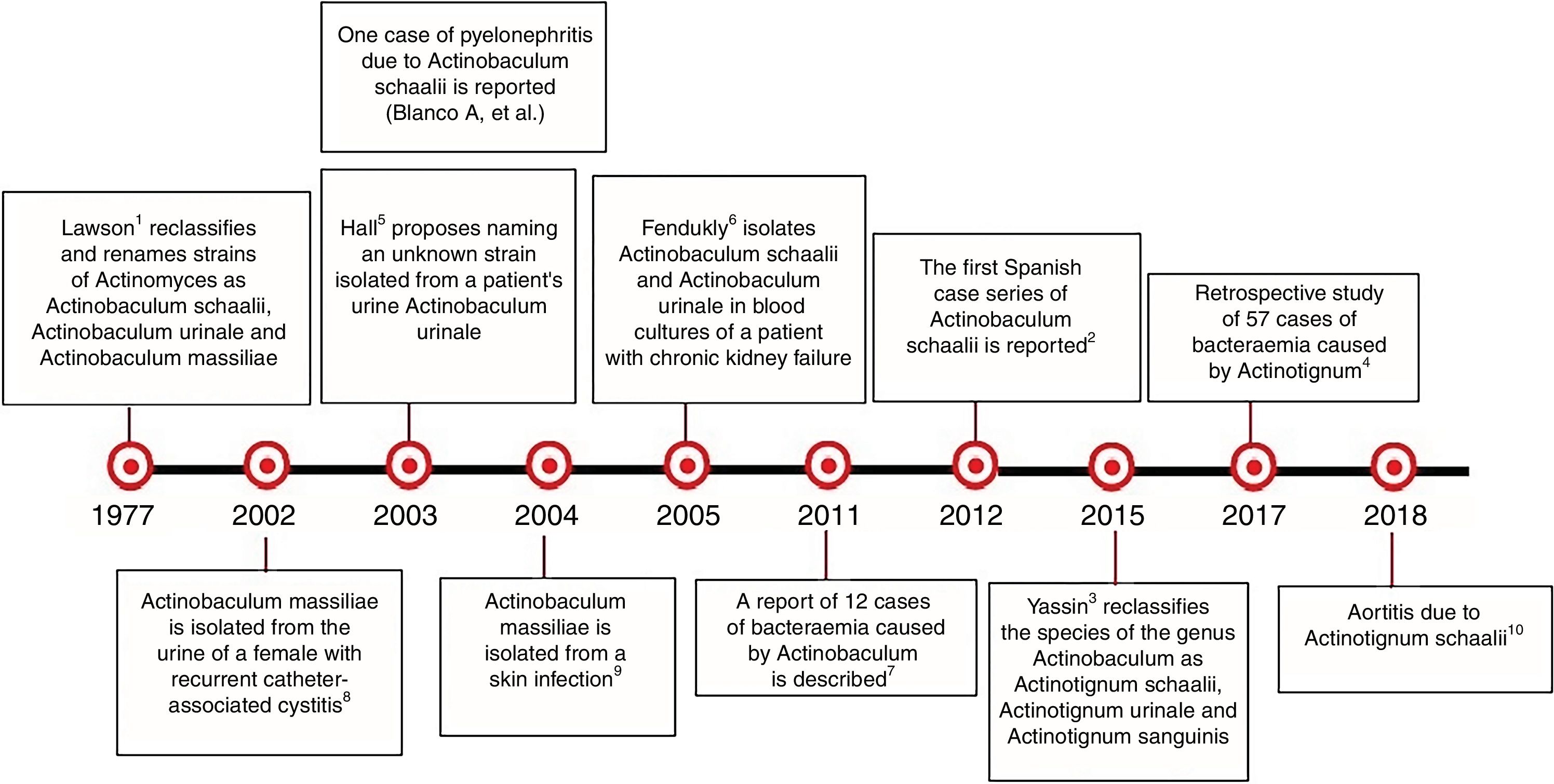
The genus Actinobaculum was first described by Lawson et al.1 in 1997, who differentiated it from Actinomyces. It includes the species Actinobaculum suis (swine pathogen), Actinobaculum schaalii, A. urinale and Actinobaculum massiliae, which have been linked to urinary tract infections. Cases of bacteraemia, osteomyelitis, endocarditis, arteritis, skin and soft-tissue infections and sepsis of urinary origin have been reported.2–6 In 2015, Yassin et al.7 reclassified the genus as Actinotignum, including the species A. schaalii, A. urinale and Actinotignum sanguinis. Actinotignum probably forms part of the urogenital microbiota. They are small gram-positive, catalase-negative, slightly curved, non-spore-forming and immobile coccobacilli. They are obligate or facultative anaerobes. Given their similar macroscopic appearance to microorganisms of the genus Streptococcus and the microscopic features of the genus Corynebacterium and Propionibacterium, they may be confused with microorganisms of the mucocutaneous saprophytic microbiota. The conditions in which urogenital samples are usually incubated may mean that genitourinary infections caused by these microorganisms are underdiagnosed. The use of MALDI-TOF® and molecular biology (16S rRNA gene sequencing) are essential for their identification. Failures to differentiate the species A. schaalii and A. sanguinis by 16S rRNA gene sequencing have been reported.8 In infections caused by this genus, the possibility of therapeutic failures with empirical treatments using quinolones or co-trimoxazole should be considered,8 with the use of beta-lactams being preferred.
There are very few published cases of A. urinale infection9,10 (Fig. 2). The use of MALDI-TOF® will help to diagnose infections caused by this microorganism.
Chronological history of Actinotignum.2–6
We would like to thank Dr. Pilar López García for dedicating her time and efforts to improve this article, and Dr. Juan A. Sáez for his contribution.
Please cite this article as: Parra-Grande M, Ortiz-Gorraiz MA, Abreu-di Berardino M, de la Rica-Martínez A. Hidrocele infectado. Enferm Infecc Microbiol Clin. 2019;37:341–343.