Borrelia miyamotoi is a tick-borne pathogen belonging to the relapsing fever group. It had not been reported from Spain, but its wide distribution and the presence of the tick-vector (Ixodes ricinus) made us suspect its circulation. The aim of this study was to investigate the presence of Borrelia spp. in I. ricinus in Spain.
MethodsA total of 652 I. ricinus nymphs collected in northern Spain were processed. The DNA was extracted using incubations with ammonium hydroxide. Borrelia spp. DNA was amplified using Borrelia-specific PCR assays (glpQ, 16S rRNA and flagellin genes).
ResultsB. miyamotoi was amplified in 4 specimens, and Borrelia burgdorferi sensu lato in 27 (8 Borrelia afzelii, 7 Borrelia garinii, 8 Borrelia lusitaniae, 3 Borrelia valaisiana and 1 B. burgdorferi sensu stricto).
ConclusionB. miyamotoi should be considered in the differential diagnoses of patients with confirmed or suspected tick-bite in Spanish endemic areas for Lyme disease.
Borrelia miyamotoi, patógeno del grupo de las fiebres recurrentes transmitido por garrapatas, no había sido encontrado en España. Su amplia distribución y la presencia del vector (Ixodes ricinus) nos hizo sospechar su circulación. El objeto del estudio fue investigar la presencia de Borrelia spp. en I. ricinus de España.
MétodosSe procesaron 652 ninfas recogidas en el norte de España. El ADN se extrajo mediante incubaciones de amonio. Se llevaron a cabo PCR específicas para la amplificación de ADN de Borrelia spp. (genes glpQ, ARNr 16S y flagelina).
ResultadosSe amplificó B. miyamotoi en 4 ejemplares y Borrelia burgdorferi sensu lato en 27 (8 Borrelia afzelii, 7 Borrelia garinii, 8 Borrelia lusitaniae, 3 Borrelia valaisiana y 1 Borrelia burgdorferi sensu stricto).
ConclusiónB. miyamotoi debe ser considerado en el diagnóstico diferencial de pacientes con picadura o posible picadura de garrapata en zonas endémicas de enfermedad de Lyme.
The genus Borrelia (Spirochaetes; Spirochaetales; Borreliaceae) includes two clades, Borrelia burgdorferi sensu lato and the relapsing fever complex. Borrelia miyamotoi is a tick-borne pathogen belonging to the latter group that has been recently associated with human clinical cases1. B. miyamotoi infections have been reported in North America, Asia and Europe, with human cases in The Netherlands and Germany.1–3 This spirochete is transmitted by Ixodes ticks, including Ixodes ricinus (Europe), Ixodes persulcatus (Asia) and Ixodes scapularis (North America). The microorganism has been amplified from ticks, with higher prevalence in nymphal stages, and from vertebrates in several countries.1 To date, no reports of B. miyamotoi presence have been published from Spain, but the occurrence of high populations of its vector (I. ricinus) made us suspect on its circulation. I. ricinus is the most prevalent species in the North of Spain and it is responsible for most tick-bites.4 This area is endemic for Lyme disease (LD), the most common tick-borne illness in temperate regions of the northern hemisphere with an increasing occurrence in many regions.5,6 Thus, our aim was to investigate the presence of B. miyamotoi and B. burdorgferi s.l. in I. ricinus nymphs from the North of Spain.
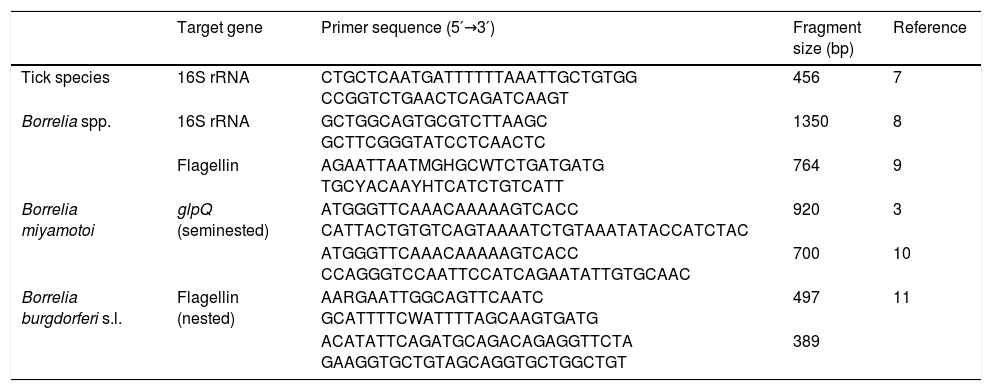
Materials and methodsTicks were selected from the collection of the Center of Rickettsiosis and Arthropod-Borne Diseases (Logroño, Spain), and chosen according to this criterion: questing I. ricinus nymphs from La Rioja and neighboring areas. Nymphs were selected because nymphal stage is the one that most frequently bites humans in the studied area4 and their highest abundance overlaps with most LD cases.5 Moreover, the highest prevalences for B. miyamotoi infection have been reported in nymphs.1 A total of 652 nymphs collected from La Rioja (n=372), Álava (n=139), Cantabria (n=17) and Navarra (n=124) were studied (Table 1). DNA was individually extracted using two incubations of 20min each with 100μL of ammonium hydroxide 0.7M at 100 and 90°C, respectively. Conventional PCR assays were performed using KAPA Taq PCR Kit (Kapa Biosystems (Pty) Ltd, SA), following manufacturer's instructions. PCRs targeting 16S rRNA mitochondrial genome of ticks were performed for all samples to validate the DNA extraction procedure. Borrelia spp. screening was performed using PCR assays for 16S rRNA and flagellin (single-run) genes. In addition, PCRs for the partial glycerophosphodiester phosphodiesterase (glpQ) gene were carried out. This gene is present in the relapsing fever complex but not in the B. burgdorferi complex. Lastly, nested PCR assays targeting a species-specific flagellin region for the B. burgdorferi s.l. detection were used (Table 2). Negative and positive controls (DNA from I. ricinus, B. miyamotoi or Borrelia spielmanii) were included in all assays. The amplicons of interest were purified with llustra™ ExoProStar™ Kit (GE Healthcare, UK). Sequencing reactions were performed using BigDye Terminator Kit (Applied Biosystems, Inc., CA) with sequence product resolution (ABI Prism 3130). Nucleotide sequences were compared with those available in NCBI database using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
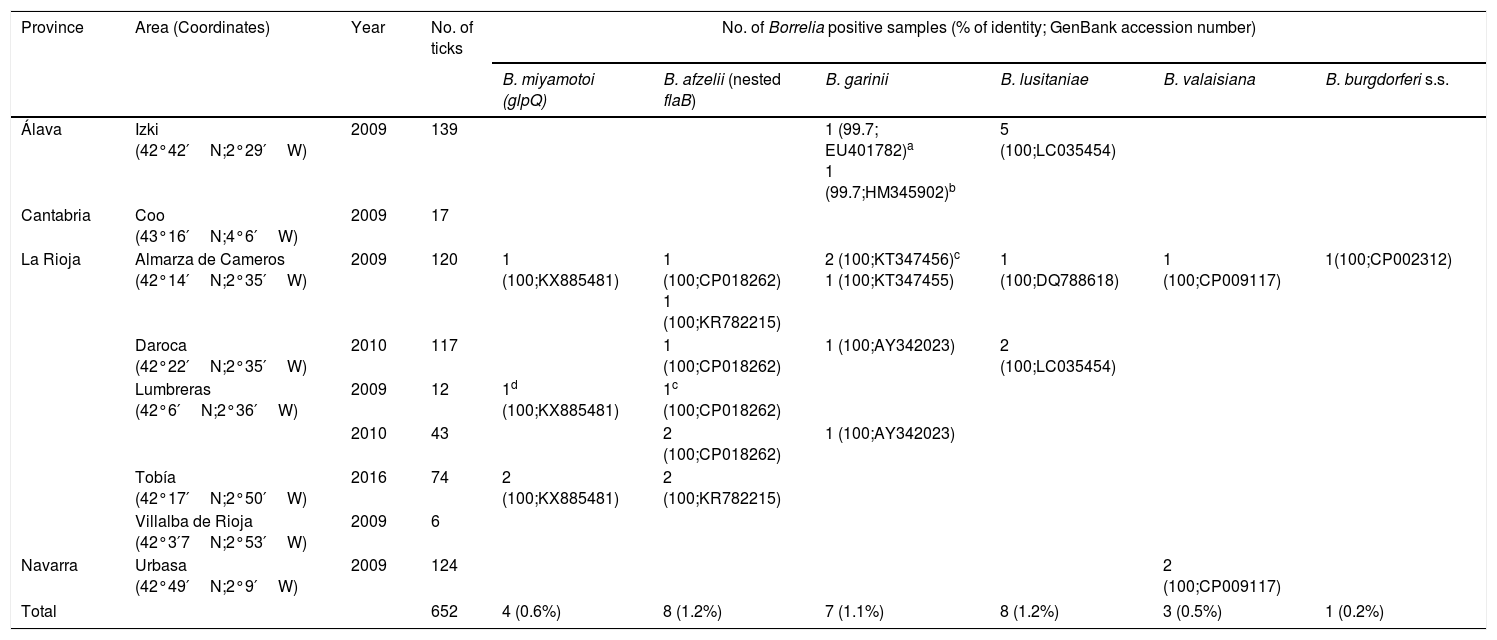
Ixodes ricinus nymphs processed in this study and Borrelia species detected.
| Province | Area (Coordinates) | Year | No. of ticks | No. of Borrelia positive samples (% of identity; GenBank accession number) | |||||
|---|---|---|---|---|---|---|---|---|---|
| B. miyamotoi (glpQ) | B. afzelii (nested flaB) | B. garinii | B. lusitaniae | B. valaisiana | B. burgdorferi s.s. | ||||
| Álava | Izki (42°42′N;2°29′W) | 2009 | 139 | 1 (99.7; EU401782)a 1 (99.7;HM345902)b | 5 (100;LC035454) | ||||
| Cantabria | Coo (43°16′N;4°6′W) | 2009 | 17 | ||||||
| La Rioja | Almarza de Cameros (42°14′N;2°35′W) | 2009 | 120 | 1 (100;KX885481) | 1 (100;CP018262) 1 (100;KR782215) | 2 (100;KT347456)c 1 (100;KT347455) | 1 (100;DQ788618) | 1 (100;CP009117) | 1(100;CP002312) |
| Daroca (42°22′N;2°35′W) | 2010 | 117 | 1 (100;CP018262) | 1 (100;AY342023) | 2 (100;LC035454) | ||||
| Lumbreras (42°6′N;2°36′W) | 2009 | 12 | 1d (100;KX885481) | 1c (100;CP018262) | |||||
| 2010 | 43 | 2 (100;CP018262) | 1 (100;AY342023) | ||||||
| Tobía (42°17′N;2°50′W) | 2016 | 74 | 2 (100;KX885481) | 2 (100;KR782215) | |||||
| Villalba de Rioja (42°3′7N;2°53′W) | 2009 | 6 | |||||||
| Navarra | Urbasa (42°49′N;2°9′W) | 2009 | 124 | 2 (100;CP009117) | |||||
| Total | 652 | 4 (0.6%) | 8 (1.2%) | 7 (1.1%) | 8 (1.2%) | 3 (0.5%) | 1 (0.2%) | ||
PCR primer pairs and conditions used in this study.
| Target gene | Primer sequence (5′→3′) | Fragment size (bp) | Reference | |
|---|---|---|---|---|
| Tick species | 16S rRNA | CTGCTCAATGATTTTTTAAATTGCTGTGG CCGGTCTGAACTCAGATCAAGT | 456 | 7 |
| Borrelia spp. | 16S rRNA | GCTGGCAGTGCGTCTTAAGC GCTTCGGGTATCCTCAACTC | 1350 | 8 |
| Flagellin | AGAATTAATMGHGCWTCTGATGATG TGCYACAAYHTCATCTGTCATT | 764 | 9 | |
| Borrelia miyamotoi | glpQ (seminested) | ATGGGTTCAAACAAAAAGTCACC CATTACTGTGTCAGTAAAATCTGTAAATATACCATCTAC | 920 | 3 |
| ATGGGTTCAAACAAAAAGTCACC CCAGGGTCCAATTCCATCAGAATATTGTGCAAC | 700 | 10 | ||
| Borrelia burgdorferi s.l. | Flagellin (nested) | AARGAATTGGCAGTTCAATC GCATTTTCWATTTTAGCAAGTGATG | 497 | 11 |
| ACATATTCAGATGCAGACAGAGGTTCTA GAAGGTGCTGTAGCAGGTGCTGGCTGT | 389 |
All samples tested positive for the partial 16S rRNA gene of ticks. Borrelia spp. were detected in 4.6% (30/652) of ticks. The glpQ nucleotide sequences corresponding to 4 I. ricinus from La Rioja were homologous to B. miyamotoi sequences from GenBank. Moreover, B. burgdorferi s.l. was amplified in 27 samples using a nested-flagellin-PCR. Sequences showed presence of Borrelia afzelii (n=8), Borrelia garinii (n=5), Borrelia lusitaniae (n=3), Borrelia valaisiana (n=1) and B. burgdorferi sensu stricto (n=1) in ticks from La Rioja. In addition, B. garinii (n=2) and B. lusitaniae (n=5) were detected in Álava and B. valaisiana (n=2) in Navarra. One tick from La Rioja showed coinfection with B. miyamotoi and B. afzelii. Only one amplicon corresponding to B. garinii was obtained with the Borrelia-specific 16S rRNA PCRs (Table 1). No samples yielded positive PCR results using a single-run flagellin PCR assay.
The sequences corresponding to B. miyamotoi found in Spain were submitted to GenBank under accession number MG136725. Sequences corresponding to B. garinii that differed from those previously included in the database were submitted to GenBank under accession numbers MG136723-24. Remaining sequences are available upon request to the authors.
DiscussionThis study demonstrates the presence of B. miyamotoi in the North of Spain, and it corroborates the circulation of B. burgdorferi genospecies (B. afzelii, B. garinii, B. lusitaniae, B. valaisiana and B. burgdorferi s.s.), all agents of LD.5,6
The complete spectrum of B. miyamotoi infection is being written. Non-European patients commonly presented a nonspecific flu-like febrile syndrome, and some of them showed an erythema migrans.1 Accordingly, we have found co-infection with B. miyamotoi and B. afzelii.
Most B. miyamotoi-related human cases have been reported from Russia, with a clinical picture of fever and malaise in almost 100% cases, followed by myalgia, chills, nausea and arthralgia.1 Patients from USA and Japan presented similar clinical characteristics. Only one immunocompromised patient from USA suffered from neurological involvement.1 Two cases have been reported in Europe, both in non-febrile immunocompromised patients with a tick-bite history. In the meningoencephalitis case reported from The Netherlands, an immunocompromised 70-year-old-man affected by a diffuse large B-cell lymphoma developed a slow cognitive process, memory deficits and disturbed gait. He had pleocytosis with raised protein values in cerebrospinal fluid (CSF) and serological assays for B. burgdorferi were inconclusive (positive C6 assay and, negative and indeterminate IgM and IgG western-blot). Treated like a Lyme neuroborreliosis, the patient recovered with 2-week-course of ceftriaxone (2g IV). B. miyamotoi infection was retrospectively confirmed using darkfield microscopy and PCRs on blood and CSF.3 In the German case, a 74-year-old-woman with a previous non-Hodgkin lymphoma developed a syndrome resembling Lyme neuroborreliosis (dizziness, vomiting and headache) with pleocytosis and raised proteins on CSF. Serological assays against B. burgdorferi were negative for serum and CSF. The infection was diagnosed by B. miyamotoi-DNA amplification on CSF. The patient recovered under prescription of ceftriaxone (2g IV; 3 weeks).2
Since there are not commercial tests available for B. miyamotoi, and serological tests for diagnosing LD are equivocal or negative, we must have a high level of suspicion for diagnosing B. miyamotoi infection. Spirochetes can be observed by darkfield microscopy or stain during the acute phase although, to date, only positive PCRs on blood or other sterile fluids like CSF can demonstrate B. miyamotoi infection. Doxycycline and ceftriaxone have been successfully prescribed.1
Herein, we have found 3 B. burgdorferi recognized pathogenic genospecies (B. afzelii, B. garinii, B. burgdorferi s.s.). The remaining genospecies amplified, B. valaisiana and B. lusitaniae, have been occasionally implicated in clinical cases, although their pathogenicity is still uncertain.4 All these genospecies had been previously reported from Spain.12–14 In this country, most LD cases are diagnosed in the North, where B. garinii was hypothesized as the main pathogen.6 Nevertheless, the high prevalence of other recognized pathogens found in this study, e.g. B. afzelii, suggests that other Borrelia species could cause LD in Spain.
According to our data, the prevalence of B. miyamotoi in ticks is lower than the one of B. burgdorferi s.l. This tendency had been previously observed.1,8 Nevertheless, the dynamics of B. miyamotoi transmission remains unknown and human cases of B. miyamotoi infection could have been undetected or misdiagnosed.
The discrepancies between the results obtained using the nested and the single-run PCRs suggest that the latter have low sensitivity, although they have been reported as useful assays for Borrelia spp. detection.8,9
In summary, we report the presence of B. burgdorferi genospecies in the vector, and the circulation in Spain of a non-previously reported tick-borne pathogen, B. miyamotoi, that must be considered in patients with neurological syndrome and/or fever after a confirmed or suspected tick-bite.
AddendumWhile we were screening ticks for B. miyamotoi detection, Dr Pablo Díaz Fernández (Facultad de Veterinaria, Universidade de Santiago de Compostela, Lugo, Spain) personally communicated the finding of B. miyamotoi in ticks from Galicia (unpublished data).
Conflict of interestsThe authors declare that they have no conflict of interest.
Partial results from this study were presented at the XXI congress of the Spanish Society of Infectious Diseases and Clinical Microbiology held in Málaga (Spain) in May 2017, and at the 4th Conference on Neglected Vectors and Vector-Borne Diseases (COST Action TD1303) held in Crete (Greece) in September 2017.
We thank Dr Volker Fingerle (German National Reference Centre for Borrelia, Oberschleissheim, Germany) and Dr Pablo Díaz (Facultad de Veterinaria, Universidade de Santiago de Compostela, Lugo, Spain) for providing Borrelia positive controls. We also thank Lourdes Romero for the support with the processing of samples.