To explore the clinical and epidemiological characteristics of chronic obstructive pulmonary disease (COPD) patients with Aspergillus spp. isolation from respiratory samples, and to identify which factors may help us to distinguish between colonisation and infection.
MethodsA retrospective cohort study was performed. All patients with COPD and respiratory isolation of Aspergillus spp. over a 12-year period were included. Patients were assigned to 2 categories: colonisation and pulmonary aspergillosis (PA), which includes the different clinical forms of aspergillosis. A binary logistic regression model was performed to identify the predictive factors of PA.
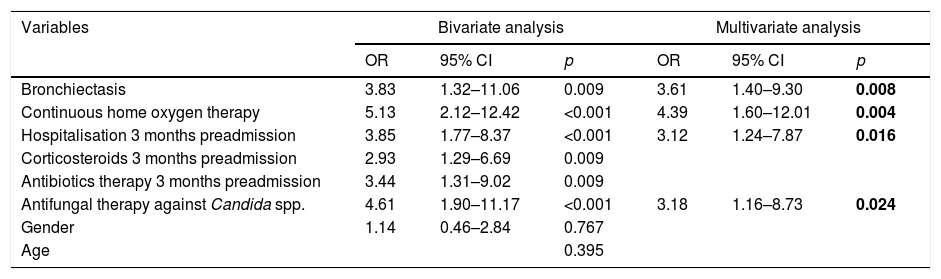
ResultsA total of 123 patients were included in the study: 48 (39.0%) with colonisation and 75 (61.0%) with PA: 68 with probable invasive pulmonary aspergillosis and 7 with chronic pulmonary aspergillosis. Spirometric stages of the GOLD classification were not correlated with a higher risk of PA. Four independent predictive factors of PA in COPD patients were identified: home oxygen therapy (OR: 4.39; 95% CI: 1.60–12.01; p=0.004), bronchiectasis (OR: 3.61; 95% CI: 1.40–9.30; p=0.008), hospital admission in the previous three months (OR: 3.12; 95% CI: 1.24–7.87; p=0.016) and antifungal therapy against Candida spp. in the previous month (OR: 3.18; 95% CI: 1.16–8.73; p=0.024).
ConclusionsContinuous home oxygen therapy, bronchiectasis, hospital admission in the previous three months and administration of antifungal medication against Candida spp. in the previous month were associated with a higher risk of pulmonary aspergillosis in patients with COPD.
Conocer las características clínicas y epidemiológicas de los pacientes con enfermedad pulmonar obstructiva crónica (EPOC) y aislamiento de especies de Aspergillus en muestra respiratoria e identificar factores que nos ayuden a diferenciar entre colonización e infección.
MétodosEstudio de cohortes retrospectivo en el que se incluyeron todos los pacientes con EPOC y aislamiento de Aspergillus spp. en muestra respiratoria durante un periodo de 12 años. Se asignaron los pacientes a 2 categorías: colonización y aspergilosis pulmonar (AP), que incluye las diferentes formas de presentación clínica. Se aplicó un modelo de regresión logística binaria para identificar los factores predictores de desarrollo de AP.
ResultadosUn total de 123 pacientes fueron incluidos en el estudio: 48 (39%) colonizados y 75 (61%) con AP: 68 con AP invasiva probable y 7 con AP crónica. No hubo correlación entre el riesgo de AP y los estadios espirométricos de la clasificación GOLD. Se identificaron como factores predictores independientes de AP en pacientes con EPOC la oxigenoterapia domiciliaria (OR: 4,39; IC 95%: 1,60-12,01; p=0,004), las bronquiectasias (OR: 3,61; IC 95%: 1,40-9,30; p=0,008), la hospitalización en los 3 meses previos al ingreso (OR: 3,12; IC 95%: 1,24-7,87; p=0,016) y la terapia antifúngica frente a Candida spp. en el mes previo (OR: 3,18; IC 95%: 1,16-8,73; p=0,024).
ConclusionesLa oxigenoterapia continua domiciliaria, las bronquiectasias, la hospitalización en los 3 meses previos al ingreso y la utilización de terapia antifúngica frente a Candida spp. en el mes previo se asocian a mayor riesgo de AP en pacientes con EPOC.
Pulmonary aspergillosis (PA) is an infection caused by a filamentous fungus of the genus Aspergillus with different forms of clinical presentation depending on the immune status and the presence of underlying lung disease: colonisation, allergic bronchopulmonary aspergillosis, non-invasive chronic lung forms and invasive forms.1,2 Invasive PA (IPA) classically affects patients with immunosuppression, such as severe neutropenia, haematological malignancies or patients who have undergone a transplantation.3,4 In recent years, an increase in the number of infections caused by this fungus has been observed in patients with a lower degree of immunosuppression, such as patients with lung diseases receiving corticosteroids, cancer, elderly individuals with prolonged hospitalisations or stays in intensive care units.5–7 Chronic obstructive pulmonary disease (COPD) that has received corticosteroids and antibiotics is the most frequent IPA risk group nowadays in hospitalised non-neutropenic patients.8 Several factors involved in the progression of COPD have been linked to an increased risk of IPA.8,9 In this group of patients, the meaning of the isolation of Aspergillus spp. in a respiratory sample is controversial. It is questioned whether colonisation could be a temporary state, a chronic carrier with a benign course or an early sign of invasive disease.10,11 Various factors such as a high percentage of colonisations, a less aggressive and less specific clinical presentation or the absence of invasive diagnostic tests, make it difficult to differentiate between fungal disease and colonisation, which implies a delay in diagnosis. Bulpa et al. have proposed the criteria of possible, probable and proven IPA in patients with COPD.12 On the other hand, we know that early diagnosis and treatment are crucial to improve the prognosis of the disease.12,13
The objective of this study was to identify factors that help us differentiate between colonisation and fungal lung disease in patients with COPD and respiratory isolate of Aspergillus spp.
Material and methodsStudy designA retrospective cohort study was conducted in an 84-bed hospital in Ribeira (A Coruña, Spain) with a reference population of 65,000 inhabitants. This hospital has a 41-bed Internal Medicine hospitalisation ward without critical or transplant units. All patients with isolation of Aspergillus spp. in a respiratory sample for a period of 12 years (from 1 January 2007 to 31 December 2018) were included. Respiratory samples were examined by Gram staining and evaluated according to the Murray and Washington criteria. Those belonging to group 4 (10–25 epithelial cells and >25leukocytes) and group 5 (<10 epithelial cells and >25 leukocytes) were considered valid.
The variables collected were: age, gender, Charlson index, comorbidities, stage of severity of COPD, clinical phenotype, previous structural lung changes, continuous home oxygen therapy (CHOt), systemic steroids and antibiotic therapy in the three months prior to admission and during the pre-isolation episode, taking nystatin or fluconazole in the previous month, hospitalisations in the previous year, exacerbations that led to admission or emergency care in the last year, days from admission to isolation, microbiological, radiological and laboratory data, antifungal treatment, clinical course and mortality.
For the assessment of the degree of comorbidity we used the Charlson comorbidity index.14 In patients with COPD, the classification was performed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines15 and the clinical phenotype according to the Spanish COPD guideline (GesEPOC).16 The use of systemic steroids was defined as taking steroids for at least 48h. Additionally, the cumulative dose and the mean daily dose of prednisone or equivalent were calculated. We defined combined broad-spectrum antibiotic therapy as the combined use of antibiotics with coverage against 3rd generation cephalosporin-resistant gram-negative bacillus and fluorquinolones and methicillin-resistant gram-positive bacteria. Respiratory samples were processed by the Microbiology Department of the reference hospital, performing standard procedures that included Gram staining and culture in Sabouraud medium for fungal isolation. The samples were incubated at between 32 and 37°C and fungal colonies were identified by macro- and microscopic methods and mass spectrometry from 2012. Serum galactomannan was determined using the Platelia Aspergillus EIA test (Bio-Rad Laboratories, France, Ref. 62794), according to the manufacturer's recommendations: an index ≥0.5 is considered positive. The Ouchterlony immunodiffusion method (Microgen Bioproducts) was used to detect serum precipitins against Aspergillus. The radiological tests were reviewed by the same radiologist to try to minimise the disparity of criteria. The patients were assigned to two categories: (a) colonisation (patients with isolation of asymptomatic Aspergillus spp. or with new respiratory symptoms with good clinical evolution with appropriate antibiotic) and (b) PA (considering the criteria of Bulpa et al.12 for IPA regardless of GOLD stage and those of Denning et al.17 for chronic forms: aspergilloma, chronic cavitary PA, chronic necrotising PA). There was no case of possible IPA (since isolation of Aspergillus spp. was required as a criterion to be included in the study). All patients were followed up until the end date of the study or death.
Statistical analysisFrequencies and percentages were calculated for qualitative variables. Quantitative variables were expressed as mean±standard deviation. The comparison of quantitative variables was performed using a Student's t-test (for variables with normal distribution) and Mann–Whitney U test (for variables with non-normal distribution). The qualitative variables were compared using the chi-square test or Fisher's exact test. The degree of association was estimated using the odds ratio (OR) with a 95% confidence interval (95% CI). A binary logistic regression analysis (successive step procedure) was performed to study which variables were independent predictors of developing lung disease due to Aspergillus spp. The presence of PA was assigned as a dependent variable and independent variables were those that presented significant differences (p≤0.05) in the bivariate analysis. The absence of significant collinearity has been confirmed through the inflation factor of the variance between the variables included in the model. To assess mortality, survival curves were drawn using the Kaplan–Meier method at the end of the follow-up period and at 120 days. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) program for Windows version 18.0 (SPSS Inc., an IBM Co. Chicago, Illinois, USA).
Ethical considerationsWe obtained the approval of the ethics committee of the centre for the use of medical data. Anonymity and confidentiality were guaranteed at all times, as well as data protection.
ResultsA total of 123 patients with isolation of Aspergillus spp. in respiratory sample were included: 48 patients were colonisations and 75 PA: 68 probable IPA, one aspergilloma, 5 chronic necrotising PA and one chronic cavitary PA. The incidence of isolation of Aspergillus spp. was one case for every 200 admissions in Internal Medicine/year and its distribution in the last 12years was similar (mean 10 cases/year). Most of the isolates were in sputum, with Aspergillus fumigatus being the most frequently isolated species in 111 patients (90.3%), followed by Aspergillus niger in nine (7.3%), Aspergillus terreus in two (1.6%) and Aspergillus spp. in eight (6.5%). The determination of serum galactomannan was performed in 34 patients (27.7%), with it being positive only in five. The mean follow-up time after isolation was 1.251days for colonisation (range 37–4.092 days) and 176 days for PA (range 1–3.603 days).
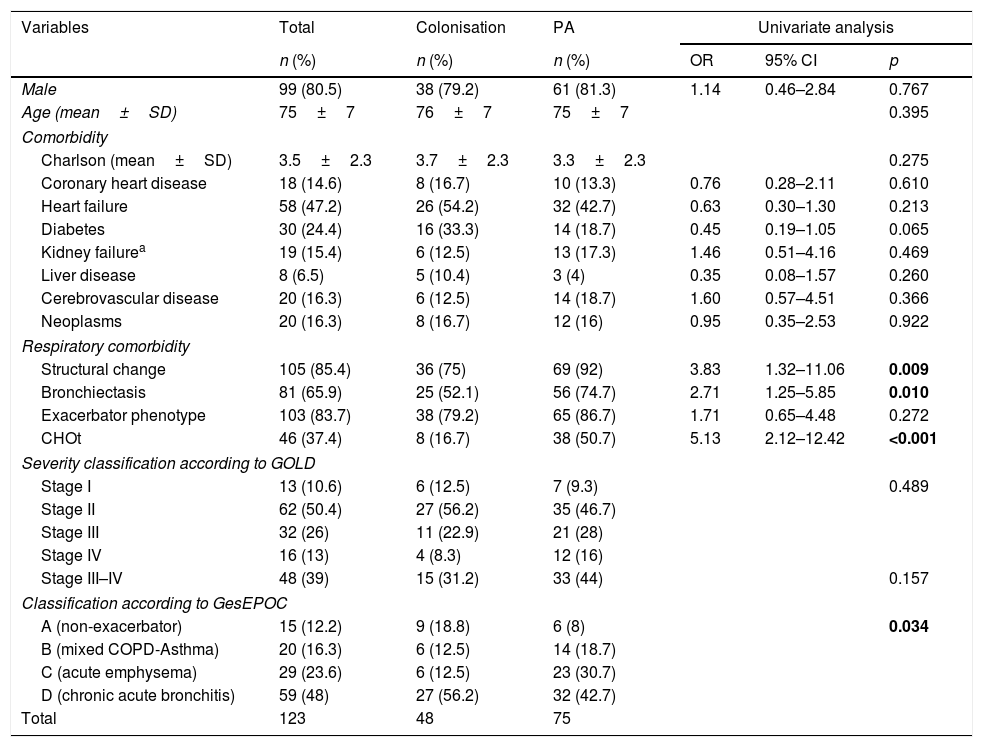
Descriptive analysisTable 1 shows the epidemiological data and comorbidities of the patients in our study. The mean age was 75 and the majority were male. Previously, 85.4% of the patients had structural lung changes (bronchiectasis 65.9%, bullae-cavities 14.6%, interstitial lung disease 8.1%, lung surgery 4.9% and pleural disease 16.3%), with them being more common in PA. No significant differences were found between the groups with respect to other comorbidities or in the severity of COPD according to the GOLD spirometric classification. In contrast, greater use of CHOt devices was observed (50.7 vs. 16.7%; p<0.001) in patients with PA.
Epidemiological data and comorbidities of the patients included in the study according to diagnostic category.
| Variables | Total | Colonisation | PA | Univariate analysis | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR | 95% CI | p | |
| Male | 99 (80.5) | 38 (79.2) | 61 (81.3) | 1.14 | 0.46–2.84 | 0.767 |
| Age (mean±SD) | 75±7 | 76±7 | 75±7 | 0.395 | ||
| Comorbidity | ||||||
| Charlson (mean±SD) | 3.5±2.3 | 3.7±2.3 | 3.3±2.3 | 0.275 | ||
| Coronary heart disease | 18 (14.6) | 8 (16.7) | 10 (13.3) | 0.76 | 0.28–2.11 | 0.610 |
| Heart failure | 58 (47.2) | 26 (54.2) | 32 (42.7) | 0.63 | 0.30–1.30 | 0.213 |
| Diabetes | 30 (24.4) | 16 (33.3) | 14 (18.7) | 0.45 | 0.19–1.05 | 0.065 |
| Kidney failurea | 19 (15.4) | 6 (12.5) | 13 (17.3) | 1.46 | 0.51–4.16 | 0.469 |
| Liver disease | 8 (6.5) | 5 (10.4) | 3 (4) | 0.35 | 0.08–1.57 | 0.260 |
| Cerebrovascular disease | 20 (16.3) | 6 (12.5) | 14 (18.7) | 1.60 | 0.57–4.51 | 0.366 |
| Neoplasms | 20 (16.3) | 8 (16.7) | 12 (16) | 0.95 | 0.35–2.53 | 0.922 |
| Respiratory comorbidity | ||||||
| Structural change | 105 (85.4) | 36 (75) | 69 (92) | 3.83 | 1.32–11.06 | 0.009 |
| Bronchiectasis | 81 (65.9) | 25 (52.1) | 56 (74.7) | 2.71 | 1.25–5.85 | 0.010 |
| Exacerbator phenotype | 103 (83.7) | 38 (79.2) | 65 (86.7) | 1.71 | 0.65–4.48 | 0.272 |
| CHOt | 46 (37.4) | 8 (16.7) | 38 (50.7) | 5.13 | 2.12–12.42 | <0.001 |
| Severity classification according to GOLD | ||||||
| Stage I | 13 (10.6) | 6 (12.5) | 7 (9.3) | 0.489 | ||
| Stage II | 62 (50.4) | 27 (56.2) | 35 (46.7) | |||
| Stage III | 32 (26) | 11 (22.9) | 21 (28) | |||
| Stage IV | 16 (13) | 4 (8.3) | 12 (16) | |||
| Stage III–IV | 48 (39) | 15 (31.2) | 33 (44) | 0.157 | ||
| Classification according to GesEPOC | ||||||
| A (non-exacerbator) | 15 (12.2) | 9 (18.8) | 6 (8) | 0.034 | ||
| B (mixed COPD-Asthma) | 20 (16.3) | 6 (12.5) | 14 (18.7) | |||
| C (acute emphysema) | 29 (23.6) | 6 (12.5) | 23 (30.7) | |||
| D (chronic acute bronchitis) | 59 (48) | 27 (56.2) | 32 (42.7) | |||
| Total | 123 | 48 | 75 | |||
CHOt: continuous home oxygen therapy; CI: confidence interval; OR: odds ratio; PA: pulmonary aspergillosis; SD: standard deviation.
Significant values are highlighted in bold.
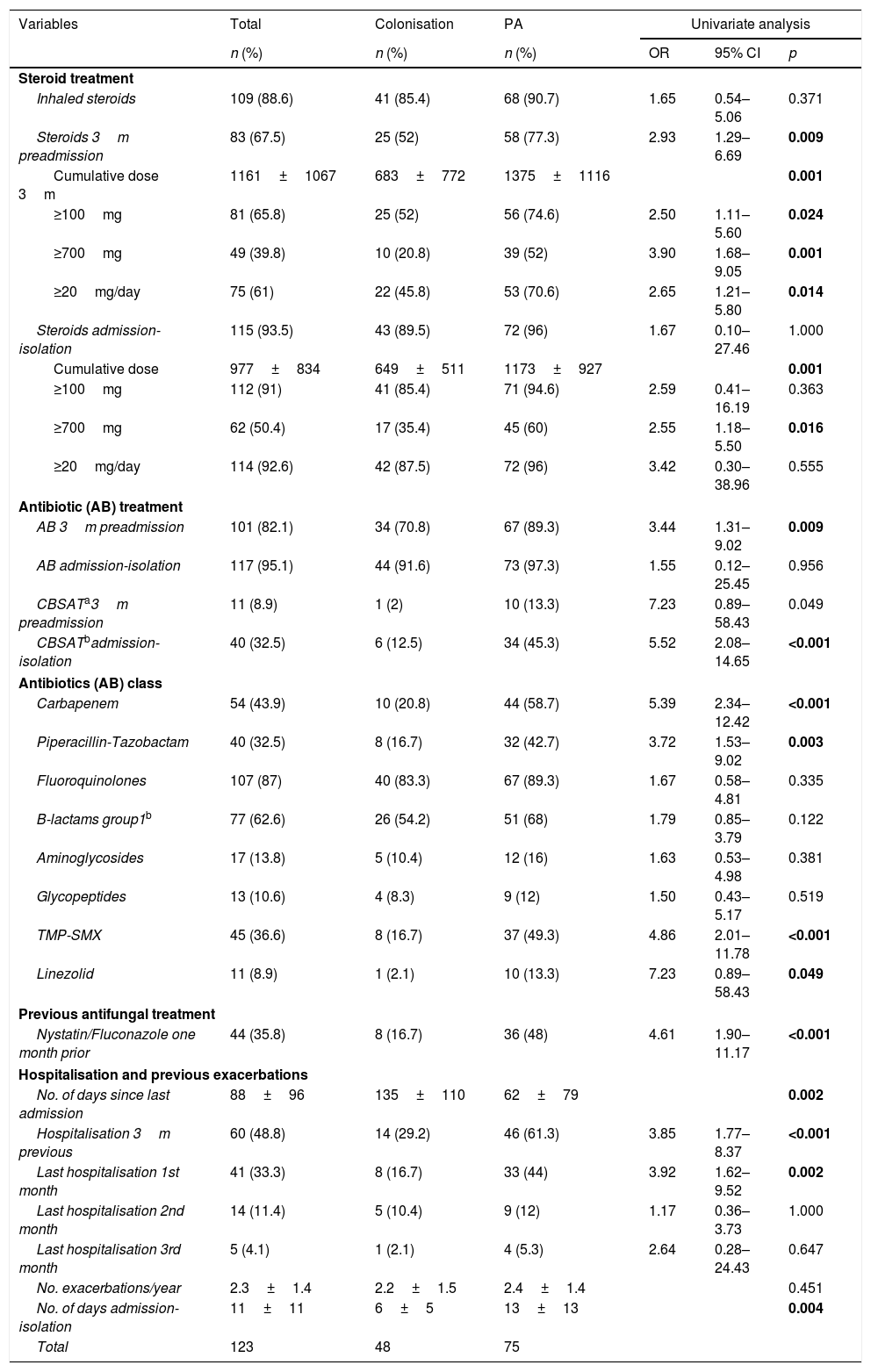
Table 2 shows the data related to the use of steroids, antibiotics, antifungals and previous hospitalisations. Patients with PA had received antibiotics and steroids more frequently in the three months prior to admission and a higher cumulative dose. Considering the period from admission to isolation, the frequency of steroid and antibiotic use is similar, but the use of broad-spectrum antibiotics and the cumulative dose of prednisone were higher in patients with PA. The use of antifungals against Candida albicans (nystatin or fluconazole) in the previous month was also higher in patients with PA. The percentage of patients hospitalised in the three months prior to admission was higher in patients with PA, although there were no differences in the number of exacerbations in the previous year.
Risk factors included in the study according to diagnostic category.
| Variables | Total | Colonisation | PA | Univariate analysis | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR | 95% CI | p | |
| Steroid treatment | ||||||
| Inhaled steroids | 109 (88.6) | 41 (85.4) | 68 (90.7) | 1.65 | 0.54–5.06 | 0.371 |
| Steroids 3m preadmission | 83 (67.5) | 25 (52) | 58 (77.3) | 2.93 | 1.29–6.69 | 0.009 |
| Cumulative dose 3m | 1161±1067 | 683±772 | 1375±1116 | 0.001 | ||
| ≥100mg | 81 (65.8) | 25 (52) | 56 (74.6) | 2.50 | 1.11–5.60 | 0.024 |
| ≥700mg | 49 (39.8) | 10 (20.8) | 39 (52) | 3.90 | 1.68–9.05 | 0.001 |
| ≥20mg/day | 75 (61) | 22 (45.8) | 53 (70.6) | 2.65 | 1.21–5.80 | 0.014 |
| Steroids admission-isolation | 115 (93.5) | 43 (89.5) | 72 (96) | 1.67 | 0.10–27.46 | 1.000 |
| Cumulative dose | 977±834 | 649±511 | 1173±927 | 0.001 | ||
| ≥100mg | 112 (91) | 41 (85.4) | 71 (94.6) | 2.59 | 0.41–16.19 | 0.363 |
| ≥700mg | 62 (50.4) | 17 (35.4) | 45 (60) | 2.55 | 1.18–5.50 | 0.016 |
| ≥20mg/day | 114 (92.6) | 42 (87.5) | 72 (96) | 3.42 | 0.30–38.96 | 0.555 |
| Antibiotic (AB) treatment | ||||||
| AB 3m preadmission | 101 (82.1) | 34 (70.8) | 67 (89.3) | 3.44 | 1.31–9.02 | 0.009 |
| AB admission-isolation | 117 (95.1) | 44 (91.6) | 73 (97.3) | 1.55 | 0.12–25.45 | 0.956 |
| CBSATa3m preadmission | 11 (8.9) | 1 (2) | 10 (13.3) | 7.23 | 0.89–58.43 | 0.049 |
| CBSATbadmission-isolation | 40 (32.5) | 6 (12.5) | 34 (45.3) | 5.52 | 2.08–14.65 | <0.001 |
| Antibiotics (AB) class | ||||||
| Carbapenem | 54 (43.9) | 10 (20.8) | 44 (58.7) | 5.39 | 2.34–12.42 | <0.001 |
| Piperacillin-Tazobactam | 40 (32.5) | 8 (16.7) | 32 (42.7) | 3.72 | 1.53–9.02 | 0.003 |
| Fluoroquinolones | 107 (87) | 40 (83.3) | 67 (89.3) | 1.67 | 0.58–4.81 | 0.335 |
| B-lactams group1b | 77 (62.6) | 26 (54.2) | 51 (68) | 1.79 | 0.85–3.79 | 0.122 |
| Aminoglycosides | 17 (13.8) | 5 (10.4) | 12 (16) | 1.63 | 0.53–4.98 | 0.381 |
| Glycopeptides | 13 (10.6) | 4 (8.3) | 9 (12) | 1.50 | 0.43–5.17 | 0.519 |
| TMP-SMX | 45 (36.6) | 8 (16.7) | 37 (49.3) | 4.86 | 2.01–11.78 | <0.001 |
| Linezolid | 11 (8.9) | 1 (2.1) | 10 (13.3) | 7.23 | 0.89–58.43 | 0.049 |
| Previous antifungal treatment | ||||||
| Nystatin/Fluconazole one month prior | 44 (35.8) | 8 (16.7) | 36 (48) | 4.61 | 1.90–11.17 | <0.001 |
| Hospitalisation and previous exacerbations | ||||||
| No. of days since last admission | 88±96 | 135±110 | 62±79 | 0.002 | ||
| Hospitalisation 3m previous | 60 (48.8) | 14 (29.2) | 46 (61.3) | 3.85 | 1.77–8.37 | <0.001 |
| Last hospitalisation 1st month | 41 (33.3) | 8 (16.7) | 33 (44) | 3.92 | 1.62–9.52 | 0.002 |
| Last hospitalisation 2nd month | 14 (11.4) | 5 (10.4) | 9 (12) | 1.17 | 0.36–3.73 | 1.000 |
| Last hospitalisation 3rd month | 5 (4.1) | 1 (2.1) | 4 (5.3) | 2.64 | 0.28–24.43 | 0.647 |
| No. exacerbations/year | 2.3±1.4 | 2.2±1.5 | 2.4±1.4 | 0.451 | ||
| No. of days admission-isolation | 11±11 | 6±5 | 13±13 | 0.004 | ||
| Total | 123 | 48 | 75 | |||
CI: confidence interval; mg: milligrams; OR: odds ratio; PA: pulmonary aspergillosis; TMP-SMX: trimethoprim-sulfamethoxazole; 3m: 3 months; 1m: 1 month.
Significant values are highlighted in bold.
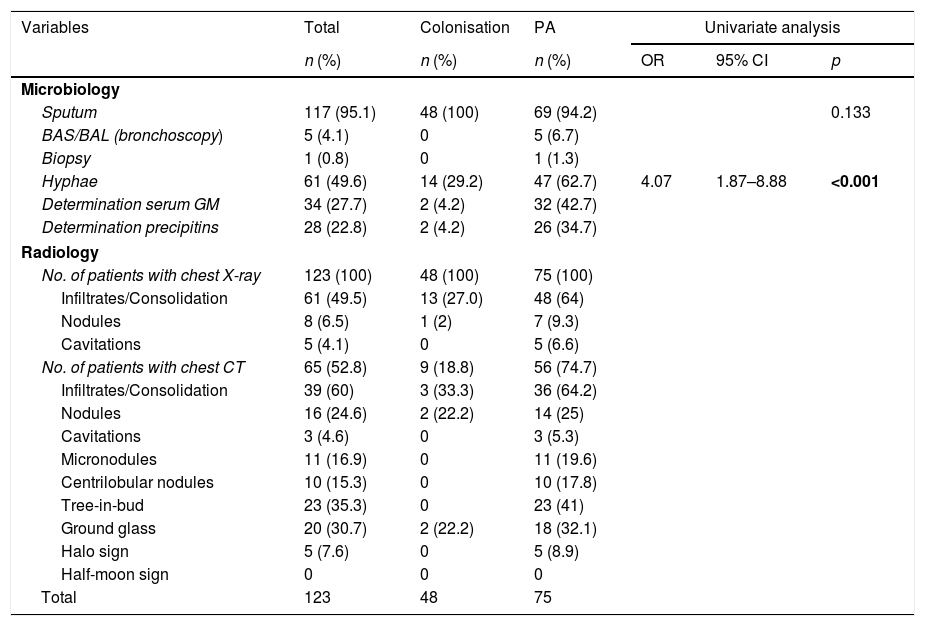
Table 3 shows the diagnostic studies performed. Regarding the analytical parameters, greater leukocytosis was observed (64 vs. 41.6%; p=0.020), increased ESR (42 vs. 24mm/h; p=0.091) and hyponatraemia (29.3 vs. 12.5; p=0.034) in the PA group without differences in haemoglobin, platelets, C-reactive protein, creatinine, lactate dehydrogenase and albumin. The presence of hyphae was higher in PA (62.7 vs. 29.2%; OR: 4.07; 95% CI: 1.87–8.88; p<0.001) and the coexistence with other microorganisms in the episode was similar in both groups (53.3 vs. 43.8%; p=0.300) with the most frequently isolated pathogens being: Stenotrophomonas maltophilia, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus and Escherichia coli.
Diagnostic procedures performed.
| Variables | Total | Colonisation | PA | Univariate analysis | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR | 95% CI | p | |
| Microbiology | ||||||
| Sputum | 117 (95.1) | 48 (100) | 69 (94.2) | 0.133 | ||
| BAS/BAL (bronchoscopy) | 5 (4.1) | 0 | 5 (6.7) | |||
| Biopsy | 1 (0.8) | 0 | 1 (1.3) | |||
| Hyphae | 61 (49.6) | 14 (29.2) | 47 (62.7) | 4.07 | 1.87–8.88 | <0.001 |
| Determination serum GM | 34 (27.7) | 2 (4.2) | 32 (42.7) | |||
| Determination precipitins | 28 (22.8) | 2 (4.2) | 26 (34.7) | |||
| Radiology | ||||||
| No. of patients with chest X-ray | 123 (100) | 48 (100) | 75 (100) | |||
| Infiltrates/Consolidation | 61 (49.5) | 13 (27.0) | 48 (64) | |||
| Nodules | 8 (6.5) | 1 (2) | 7 (9.3) | |||
| Cavitations | 5 (4.1) | 0 | 5 (6.6) | |||
| No. of patients with chest CT | 65 (52.8) | 9 (18.8) | 56 (74.7) | |||
| Infiltrates/Consolidation | 39 (60) | 3 (33.3) | 36 (64.2) | |||
| Nodules | 16 (24.6) | 2 (22.2) | 14 (25) | |||
| Cavitations | 3 (4.6) | 0 | 3 (5.3) | |||
| Micronodules | 11 (16.9) | 0 | 11 (19.6) | |||
| Centrilobular nodules | 10 (15.3) | 0 | 10 (17.8) | |||
| Tree-in-bud | 23 (35.3) | 0 | 23 (41) | |||
| Ground glass | 20 (30.7) | 2 (22.2) | 18 (32.1) | |||
| Halo sign | 5 (7.6) | 0 | 5 (8.9) | |||
| Half-moon sign | 0 | 0 | 0 | |||
| Total | 123 | 48 | 75 | |||
BAL: bronchoalveolar lavage; BAS: bronchial aspirate; CI: confidence interval; CT: computed tomography GM: galactomannan; No.: number; OR: odds ratio; PA: pulmonary aspergillosis; Rx: X-ray.
Significant values are highlighted in bold.
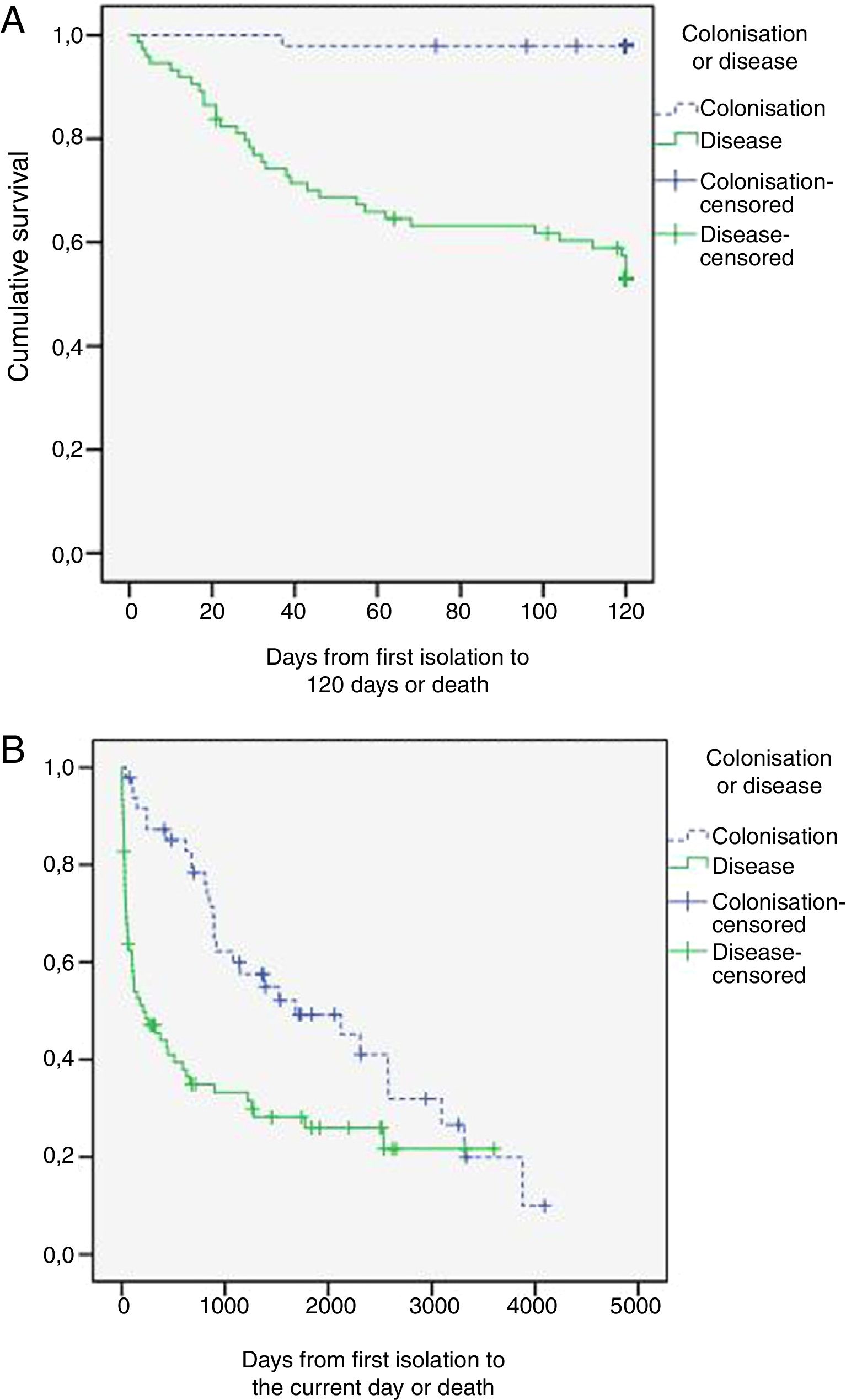
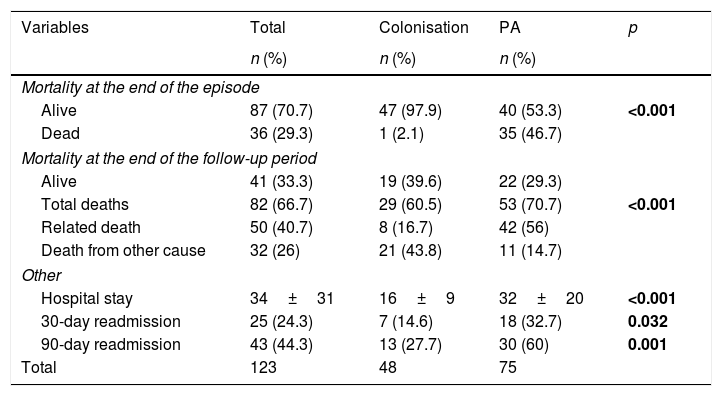
Table 4 shows the progression of the patients included in the study. Of the patients with PA, no antifungal treatment was performed in 11 patients (six diagnostic errors, four postmortem results and one non-treatment decision); they all died. The rest received voriconazole alone (39 patients; 52%), voriconazole with switch to itraconazole (10 patients; 13.3%), caspofungin or amphotericin with switch to voriconazole (4 patients; 5.4%) or itraconazole alone (11patients; 14.7%). The delay in the start of treatment was 11+17 days (median 5 days). Fig. 1 shows the survival curves at 120 days and at the end of the follow-up period. Mortality related to Aspergillus spp. at the end of the episode was higher in the PA group (46.7 vs. 2.1%; p<0.001) as was the hospital stay and readmissions within three months after discharge.
Evolution of patients included in the study.
| Variables | Total | Colonisation | PA | p |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Mortality at the end of the episode | ||||
| Alive | 87 (70.7) | 47 (97.9) | 40 (53.3) | <0.001 |
| Dead | 36 (29.3) | 1 (2.1) | 35 (46.7) | |
| Mortality at the end of the follow-up period | ||||
| Alive | 41 (33.3) | 19 (39.6) | 22 (29.3) | |
| Total deaths | 82 (66.7) | 29 (60.5) | 53 (70.7) | <0.001 |
| Related death | 50 (40.7) | 8 (16.7) | 42 (56) | |
| Death from other cause | 32 (26) | 21 (43.8) | 11 (14.7) | |
| Other | ||||
| Hospital stay | 34±31 | 16±9 | 32±20 | <0.001 |
| 30-day readmission | 25 (24.3) | 7 (14.6) | 18 (32.7) | 0.032 |
| 90-day readmission | 43 (44.3) | 13 (27.7) | 30 (60) | 0.001 |
| Total | 123 | 48 | 75 | |
PA: pulmonary aspergillosis.
Significant values are highlighted in bold.
During the follow-up of the colonised patients, PA was revealed in 21.2% (nine probable IPA and one chronic necrotising PA) with a mortality attributed to this cause of 70%, with the mean time since the index episode being 648 days (range 394–3.347 days).
Factors associated with lung disease due to Aspergillus spp.: multivariate analysisIn our study we found that CHOt (OR: 4.39; 95% CI: 1.60–12.01; p=0.004), bronchiectasis (OR: 3.61; 95% CI: 1.40–9.30; p=0.008), hospitalisation in the three months prior to admission (OR: 3.12; 95% CI: 1.24–7.87; p=0.016) and antifungal therapy against Candida spp. in the previous month (OR: 3.18; 95% CI: 1.16–8.73; p=0.024) are statistically significant and independently associated with the presence of lung disease due to Aspergillus spp. (Table 5).
Variables included in the multivariate analysis to predict pulmonary aspergillosis.
| Variables | Bivariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Bronchiectasis | 3.83 | 1.32–11.06 | 0.009 | 3.61 | 1.40–9.30 | 0.008 |
| Continuous home oxygen therapy | 5.13 | 2.12–12.42 | <0.001 | 4.39 | 1.60–12.01 | 0.004 |
| Hospitalisation 3 months preadmission | 3.85 | 1.77–8.37 | <0.001 | 3.12 | 1.24–7.87 | 0.016 |
| Corticosteroids 3 months preadmission | 2.93 | 1.29–6.69 | 0.009 | |||
| Antibiotics therapy 3 months preadmission | 3.44 | 1.31–9.02 | 0.009 | |||
| Antifungal therapy against Candida spp. | 4.61 | 1.90–11.17 | <0.001 | 3.18 | 1.16–8.73 | 0.024 |
| Gender | 1.14 | 0.46–2.84 | 0.767 | |||
| Age | 0.395 | |||||
CI: confidence interval; OR: odds ratio.
Significant values are highlighted in bold.
Aspergillus spp. lung infections in patients with COPD can be serious, reaching mortality figures similar to neutropenic patients according to the series.18 On the other hand, the significance of an isolation of Aspergillus spp. in a respiratory sample remains controversial. The high comorbidity and fragile situation of COPD patients makes it difficult to perform invasive tests and this forces us to look for other factors that help us differentiate between colonisation and PA in order to make an early diagnosis. In our study, CHOt, bronchiectasis, hospitalisation in the three months prior to admission and the use of nystatin or fluconazole in the previous month are independent risk factors for PA.
Recent studies have shown that sputum samples or bronchial secretions are just as useful for demonstrating IPA as those obtained by BAL.19 Bouza et al. observed that the probability of IPA increased with the number of samples in which the fungus was isolated.11 In our study, most of the samples were sputum, with the average being 2.6 positive samples.
Another important aspect is knowing which patients with COPD have a higher risk. The existence of a structural lung change can be an important substrate for Aspergillus spp. We have observed that the presence of bronchiectasis is greater in patients with PA, with a risk 3.6times greater. Previous studies have also indicated that patients with more severe COPD have a greater susceptibility to developing PA.20,21 However, we have not observed these differences when using the GOLD 2017 classification, based on spirometry, but the use of CHOt is more frequent among those who are diagnosed with PA. This could be explained because in the previous classifications chronic respiratory failure was included directly in stage iv, regardless of FEV1. It is possible that other indications of oxygen therapy coexist, which are a confounding factor, so we consider that specific studies are necessary.
On this substrate, a patient with COPD with structural pathology, other factors that favour the proliferation of Aspergillus spp. such as steroids, could act. Some authors have reported that steroid treatment is associated with an increased risk of PA.8,22,23 In our study, this is so when considering the three months prior to admission; however, there is no difference between the groups during the pre-isolation admission and this variable is not supported in the multivariate analysis. We believe that this may be due to the high frequency of steroid administration in patients with COPD exacerbation. The cumulative doses in both periods are also higher in patients with PA. Another risk factor discussed is the use of prior antibiotic therapy.8,23 We have seen that patients with PA have received antibiotics more frequently in the three months prior to admission. On the other hand, during the index episode there are no differences in the overall use of antibiotics, but in terms of type, and it is more frequent in patients with PA who have received broad-spectrum antibiotics. It is complex to interpret these data, because we cannot attribute causality, and it could be a reflection of the poor clinical evolution of the patient, which led to antibiotic escalation prior to the isolation of Aspergillus spp.
Other factors such as hospitalisation in the three months prior to admission or the use of nystatin or fluconazole in the previous month have shown significant differences, with a three-fold greater risk of presenting PA. These patients often require prolonged hospitalisations and frequent use of corticosteroids and antibiotics. This can cause the appearance of Candida spp. infections and, consequently, the use of nystatin or fluconazole, both without activity against Aspergillus spp. The use of these drugs could cause a decrease in colonisation by Candida spp. thus favouring the proliferation of Aspergillus spp., as has been shown in other studies.24,25
Typical radiological abnormalities of IPA are rare in these patients. Stergiopoulou et al. have described the histological lesions that explain the radiological alterations (infiltrates and nodules without the halo sign) observed in COPD patients receiving corticosteroids.26 In our study, the most frequent radiological alterations were infiltrates and nodules; we only found the halo sign in five patients.
Another important aspect to highlight is the high frequency of association with other pathogens already described by other authors.8 In 40 (53.3%) of the 75 patients with PA another pathogen was isolated, with the appropriate antibiotic and poor clinical response.
Mortality in our series reached figures of 46.7% at 120 days, increasing to 56% when we prolonged the follow-up. It should be noted that all cases that did not receive antifungal treatment died and that 21.2% of the colonised cases developed PA in subsequent years with an attributable mortality of 70%. Therefore, we believe that an early start of treatment with effective drugs, such as voriconazole, once the suspicion is established, in these patients and a follow-up of colonised patients is important, especially if other risk factors for PA are added.
Strengths and limitationsOur study presents the limitations of a retrospective study where the results depend on the quality of the data collected. Perhaps the most important limitation is that most patients have no histological confirmation and isolation was done in sputum samples. This could lead to errors in the categorisation of patients. Another limitation is that not all patients have had CT and galactomannan performed during the episode, which makes it difficult to speculate about their value in the diagnosis of PA.
As strengths, it should be noted that they are patients with COPD, a population at risk that is becoming increasingly important and is little studied, and the size of the series is considerable. In addition, the factors found are clinical factors that are easy to obtain due to the clinical history without the need for invasive procedures in patients with high comorbidity.
ConclusionsIn patients with exacerbation of COPD and isolation of Aspergillus spp. in a respiratory sample that does not improve despite adequate antibiotic and steroid treatment, CHOt, bronchiectasis, hospitalisation in the three months prior to admission and the use of antifungals against Candida spp. in the previous month there is an association with an increased risk of presenting PA. Thus, with a patient in these circumstances, we should suspect infection by said fungus and consider starting an antifungal treatment early.
FundingThis research did not receive specific financial assistance from public sector agencies, the commercial sector or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Molinos-Castro S, Pesqueira-Fontán PM, Rodríguez-Fernández S, Rodríguez-Framil M, Barbeito-Castiñeiras G, Gayol-Fernández MC, et al. Factores clínicos asociados a enfermedad pulmonar por Aspergillus spp. en pacientes con enfermedad pulmonar obstructiva crónica. Enferm Infecc Microbiol Clin. 2019;2020:4–10.