Salmonella infections (SI) are common in Spain. The aim of this study was to appraise risk factors and the clinical characteristics of sporadic Salmonella Typhimurium infections compared with other sporadic salmonella serotype infections (OSI).
MethodsFrom September 2014 to August 2015, a case-case study was carried out by the Epidemiology Division of the Public Health Centre of Castellon. Case 1 consisted of patients with sporadic S. Typhimurium infections, while case 2 comprised OSI patients, assessed according to the stool cultures analyzed by the Microbiology Laboratories of Hospital General de Castellon and Hospital de La Plana in Vila-real. Patients from detected outbreaks were not included. The salmonella serotype was identified by the National Centre of Microbiology (Madrid).
ResultsThe total number of SI patients reported was 327, 242 of whom were studied (74.0%). 148 patients had sporadic S. Typhimurium infection and 64 had OSI, with median ages of 4 and 8.5 years, respectively. Sporadic S. Typhimurium infection patients presented more blood in feces and diarrhea episodes. Consumption of pork meat (OR=2.22; 95% CI 1.12–4.43), cold pork meats (OR=2.49; 95% CI 1.32–4.68) and playing in the dirt (OR=3.02; 95% CI 1.55–5.88), were associated with sporadic S. Typhimurium infection. In the 0–4 year-old group, the associated factors were consumption of cold pork meats, omelets and female gender. In the 5-year-old and over group, only playing in soil was associated with sporadic S. Typhimurium infection.
ConclusionsThe consumption of pork and omelets, as well as playing in the dirt, were the main factors associated with infection. Children were most affected by sporadic S. Typhimurium infection.
Las infecciones por Salmonella son frecuentes en España. El objetivo de este estudio fue estimar factores de riesgo y características de las infecciones esporádicas por Salmonella typhimurium (IET), comparadas con infecciones esporádicas por otros serotipos de Salmonella.
MétodosDesde septiembre de 2014 a agosto de 2015 se efectuó un estudio caso-caso por la Sección de Epidemiología del Centro de Salud Pública de Castellón. El caso 1 fueron los pacientes con IET y el caso 2 aquellos con infecciones esporádicas por otros serotipos de Samonella, según los coprocultivos realizados por los laboratorios de Microbiología de los hospitales General de Castellón y La Plana de Vila-real, sin incluir los pacientes de brotes detectados. El serotipo de Salmonella fue identificado en el Centro Nacional de Microbiología (Madrid).
ResultadosSe notificaron 327 pacientes con infección por Salmonella, de los que 242 fueron estudiados (74,0%). Ciento cuarenta y ocho pacientes tenían IET y 64, infecciones esporádicas por otros serotipos de Salmonella, con una mediana de edad de 4 y 8,5 años, respectivamente. Los pacientes IET presentaron más episodios diarreicos y sangre en las heces. El consumo de carne de cerdo (OR 2,22; IC 95% 1,12-4,43), fiambres de cerdo (OR 2,49; IC 95% 1,32-4,68) y jugar en la tierra (OR 3,02; IC 95% 1,55-5,88) fueron asociados con IET. En el grupo de 0-4 años, los factores asociados fueron consumo de fiambres de cerdo, tortillas y ser mujer. En el grupo de 5 y más años, solo jugar en la tierra fue asociado con IET.
ConclusionesEl consumo de productos porcinos y tortillas, así como jugar en la tierra fueron los factores asociados, y los niños, los más afectados.
Sporadic cases and outbreaks of Salmonella infections continue to be frequent in Spain.1 During 2014, 241 food-borne outbreaks (FBO) of Salmonella with 1681 cases were notified in Spain, 10 FBO with 242 cases in the Valencia Community (VC), and 3 FBO with 25 cases in Castellon Health Department.2 The incidence rates were 3.6, 4.8 and 5.2 per 100,000 inhabitants, respectively. However, in 2014 the numbers of notified cases with positive Salmonella culture were 7288 in Spain and 291 in Castellon with rates of 15.6 and 60.9 per 100,000 inhabitants, respectively.2,3 These data reflect only limited morbidity of salmonellosis considering the iceberg phenomenon of mandatory notified diseases. In addition, episodes of community FBO Salmonella continued to occur in Castellon.4 From studies of Salmonella epidemiology in Castellon in previous years, a change can now be detected with S. Typhimurium currently being the most common serotype isolated in culture compared to Salmonella Enteritidis.5,6
The transmission of Salmonella follows a fecal–oral route and the consumption of foods, more rarely water, contaminated with the bacteria, is the most frequent vehicle of infection. Knowledge of the epidemiologic and the microbiological characteristics of the Salmonella species in the territory is the first step in preventing these infections. In this context, several epidemiologic designs could be used such as the case-case design,7 and the study of isolated Salmonella by serotype, phage-type, and molecular methods including pulsed-field gel electrophoresis and whole-genome sequencing may provide a deeper knowledge.
The aim of this study was to find associated factors and clinical characteristics of sporadic S. Typhimurium infections compare with other sporadic Salmonella serotypes infections using a case-case design in order to establish more appropriate measures of prevention.
Material and methodsIn 2014, mandatory reporting of Salmonella was introduced in the VC, and results of positive Salmonella culture from the microbiology laboratories of public hospitals (MLPH) were sent to public health center epidemiology units. All positive Salmonella culture patients were then interviewed about the characteristics of the disease and potential risk factors as part of the epidemiologic surveillance of this infection. In addition, the MLPH sent Salmonella isolated in the culture to the Salmonella National Reference Laboratory (SNRL) of the Instituto Carlos III in Majadahonda (Madrid) to identify the serotyping and phage-typing.
From September 2014 to August 2015 a case-case study was carried out with all positive Salmonella cultures. These cases were divided in two groups: case1 as positive S. Typhimurium status and case2 as positive other Salmonella serotypes status. To obtain enhanced comparisons of patients only culture of feces was considered, and patients from detected outbreaks were not included. Staff at the Epidemiologic Division of the Public Health Center in Castellon contacted physicians to ask about the culture and request authorization to interview the patients. The patients or their parents were then contacted by telephone and their oral informed consent was obtained. The ethic committee of the Hospital General of Castellon approved this study.
The interview was the same for case 1 and case 2 patients and was carried out at which a mean of 61.1±38.9 days from the Salmonella infections notification. The questionnaire asked about the clinical characteristics of the disease, its evolution, and risk factors including habitual food consumption, focusing on meat, chicken eggs and pork products, food preparation, hygiene practices in the kitchen, taking antibiotics, contacts with animals, among others.
Microbiological methodsThe positive Salmonella culture arrived from the Microbiologic Laboratories of the Hospital General in Castellon and Hospital La Plana in Vila-real. In both Hospitals stool samples were inoculated on usual media. In the Microbiology Laboratory of the Hospital La Plana screening of suspected colonies was performed with an agglutination test (Difco Salmonella O Antiserum Poly A-I & Vi, BD). Strains with positive agglutination were identified with the automated system Walk Away (Beckman Coulter Inc.). In the Microbiology Laboratory of the Hospital General suspected colonies were directly identified with the automated system VITEK 2 (Biomérieux). They serotyped Salmonella isolates by agglutination, according to the Kauffmann-White scheme, using polyvalent and monovalent antisera to somatic and to flagellar antigens (Bio-Rad; Statens Serum Institute). It was possible to identify these different serotypes: Salmonella Typhimurium, Salmonella Enteritidis, Salmonella Typhi, Salmonella Hadar and Salmonella Virchow. The rest of Salmonella isolated were only characterized as group B, C and D or as Salmonella spp.
All Salmonella strains were also referred to the SNRL at Majadahonda (Madrid) for its phage typing and to complete its serotyping. Salmonella serotype was determined by the slide agglutination method using commercial antisera (Bio-Rad) and following the Kauffman-White scheme for serotype attribution. Phage typing for serotypes Typhimurium, biphasic and monophasic variants, Enteritidis, Virchow, Hadar and Typhi was performed according to the international schemes with phages and interpreting criteria provided by the Health Protection Agency (HPA, formerly Public Health Laboratory Service, Colindale in London, England).
Statistical analysisTwo analyses were performed: a general analysis with all the cases of Salmonella infections, and other analysis according two age groups, considering one group from 0 to 4 years old and the other of 5 years old and over. These groups may be considered appropriate since more than 50% of S. Typhimurium cases were in children under the age of 5 years old.
The comparison between case 1 and case 2 was done by Chi2 and Fisher tests for the qualitative variables and by the Kruskal–Wallis test for the quantitative variables. Logistic regression was used to find factors associated with the Salmonella infections compare by odds ratio (OR) with a 95% confidence interval (CI). Multivariate logistic regression was used to analyze the associations with several risk factors. The factors were included in the initial models if they were associated with a significant p value<0.10. The final models presented a good of fit, p value >0.05. All calculations were performed with Stata® version 14.
ResultsIn the period of the study 327 positive Salmonella cultures were notified and 242 cases were included (74.0%). Of the 21 Salmonella isolated from outbreaks, 17 were S. Typhimurium and the remaining 4 other serotypes. From feces culture, 212 Salmonella were isolated, 148 (69.3%) S. Typhimurium and 64 (30.7%) other Salmonella serotypes. Nine Salmonella were isolated from other clinical samples (blood, urine, and skin abscess).
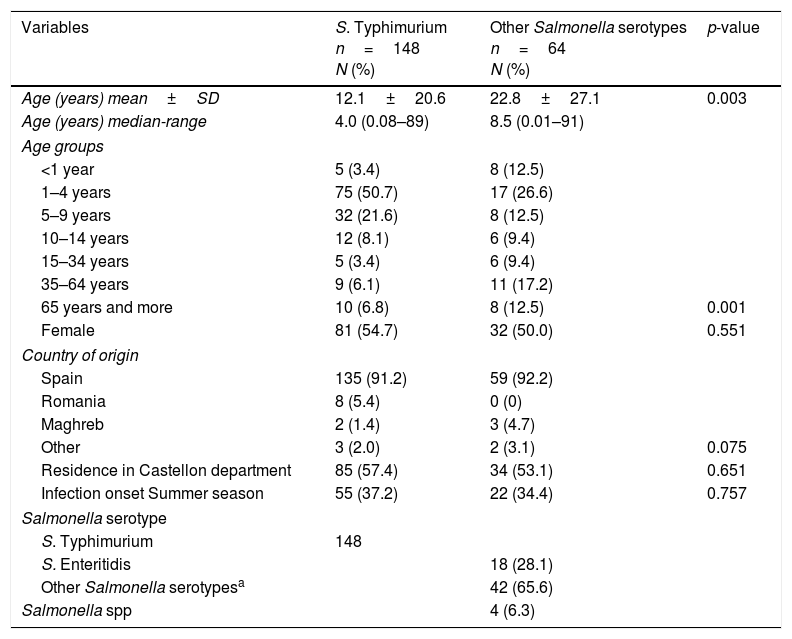
Table 1 presents the characteristics of these case 1 and case 2. The most affected age group was 1–4 years old for both case 1 and case 2. In case 1 with age median of 4.0 years, the second ranks more affected was 5–9 years old. In the case 2 with age median 8.5 years, the second group most affected was 35–65 years old. No significant differences were observed by gender, or country of origin. Other Salmonella serotypes isolated with more than one positive culture included the following: Derby (2), Rissen (7), Hadar (3), Newport (3), Mikawasima (5), Virchow (5), Goldcoast(4), Mbandaka (2), Bredeney (2) and Braenderup (2). The most frequent S. Typhimurium phage-types were DT104 (25), U311 (14), DT138 (12), RDNC(12), DT93 (11), and untypable (6).
Characteristics of sporadic S. Typhimurium and other Salmonella serotypes patients.
| Variables | S. Typhimurium n=148 N (%) | Other Salmonella serotypes n=64 N (%) | p-value |
|---|---|---|---|
| Age (years) mean±SD | 12.1±20.6 | 22.8±27.1 | 0.003 |
| Age (years) median-range | 4.0 (0.08–89) | 8.5 (0.01–91) | |
| Age groups | |||
| <1 year | 5 (3.4) | 8 (12.5) | |
| 1–4 years | 75 (50.7) | 17 (26.6) | |
| 5–9 years | 32 (21.6) | 8 (12.5) | |
| 10–14 years | 12 (8.1) | 6 (9.4) | |
| 15–34 years | 5 (3.4) | 6 (9.4) | |
| 35–64 years | 9 (6.1) | 11 (17.2) | |
| 65 years and more | 10 (6.8) | 8 (12.5) | 0.001 |
| Female | 81 (54.7) | 32 (50.0) | 0.551 |
| Country of origin | |||
| Spain | 135 (91.2) | 59 (92.2) | |
| Romania | 8 (5.4) | 0 (0) | |
| Maghreb | 2 (1.4) | 3 (4.7) | |
| Other | 3 (2.0) | 2 (3.1) | 0.075 |
| Residence in Castellon department | 85 (57.4) | 34 (53.1) | 0.651 |
| Infection onset Summer season | 55 (37.2) | 22 (34.4) | 0.757 |
| Salmonella serotype | |||
| S. Typhimurium | 148 | ||
| S. Enteritidis | 18 (28.1) | ||
| Other Salmonella serotypesa | 42 (65.6) | ||
| Salmonella spp | 4 (6.3) | ||
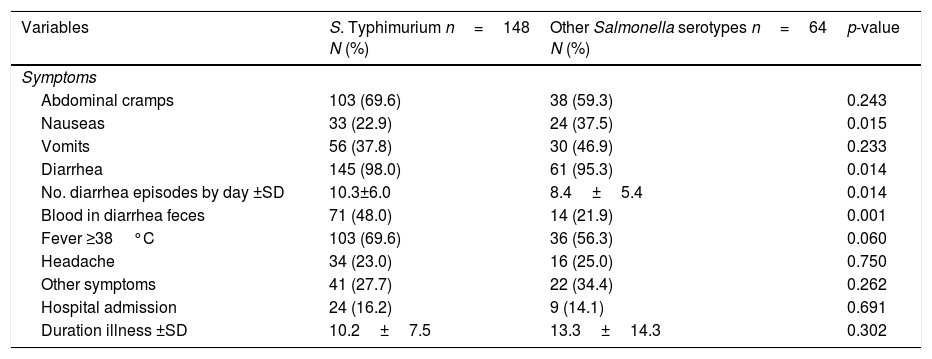
The patients suffered acute gastroenteritis and their clinical symptoms are presented in Table 2. The group case 1 suffered more significant blood in the feces and higher numbers of diarrhea episodes by day than group case 2. However, nausea was more frequent in group case 2. The significance of these symptoms held when age was adjusted for. No other significant differences were observed. The hospital admissions in the case 1 and case 2 groups were 16.2% and 14.1%, respectively. Clinical evolution was good for all the patients.
Comparison of the clinical characteristic of sporadic S. Typhimurium and other Salmonella serotypes patients.
| Variables | S. Typhimurium n=148 N (%) | Other Salmonella serotypes n=64 N (%) | p-value |
|---|---|---|---|
| Symptoms | |||
| Abdominal cramps | 103 (69.6) | 38 (59.3) | 0.243 |
| Nauseas | 33 (22.9) | 24 (37.5) | 0.015 |
| Vomits | 56 (37.8) | 30 (46.9) | 0.233 |
| Diarrhea | 145 (98.0) | 61 (95.3) | 0.014 |
| No. diarrhea episodes by day ±SD | 10.3±6.0 | 8.4±5.4 | 0.014 |
| Blood in diarrhea feces | 71 (48.0) | 14 (21.9) | 0.001 |
| Fever ≥38°C | 103 (69.6) | 36 (56.3) | 0.060 |
| Headache | 34 (23.0) | 16 (25.0) | 0.750 |
| Other symptoms | 41 (27.7) | 22 (34.4) | 0.262 |
| Hospital admission | 24 (16.2) | 9 (14.1) | 0.691 |
| Duration illness ±SD | 10.2±7.5 | 13.3±14.3 | 0.302 |
SD=standard deviation.
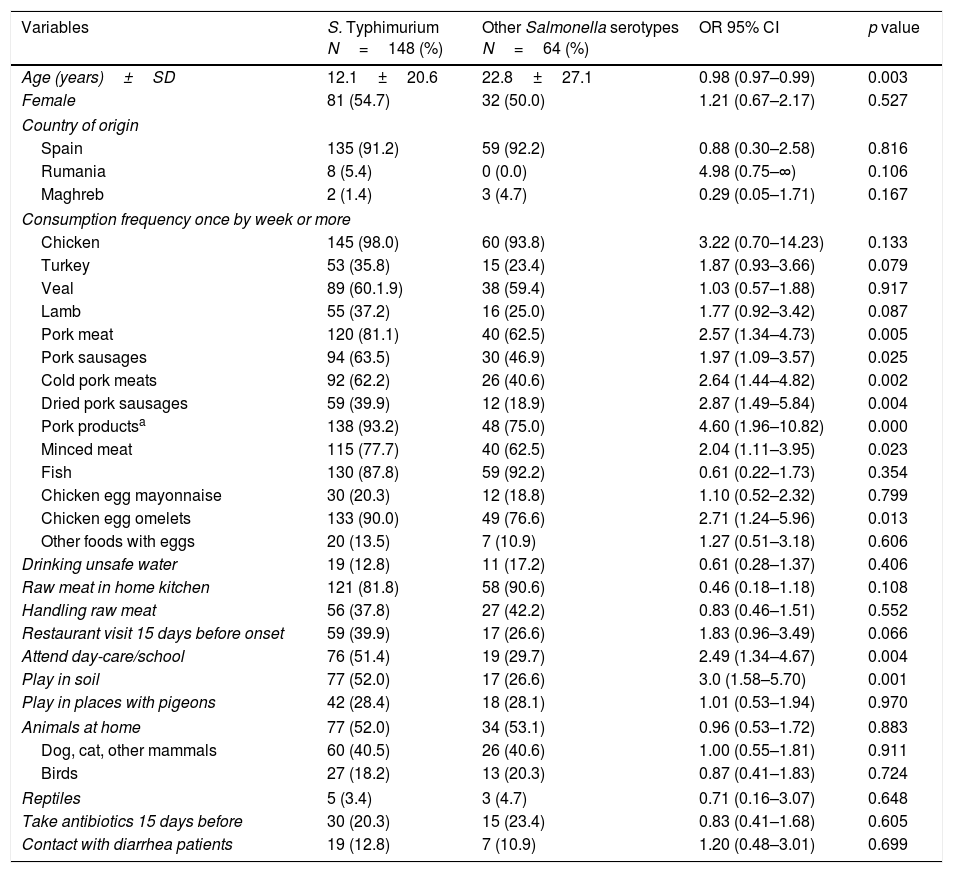
Several factors were associated with S. Typhimurium infection in the logistic regression analysis (Table 3), namely, age, consumption of some specific foods once per week or more, including pork products, pork meat, pork sausages, cold pork meats, and dried pork sausages, minced meat, and chicken egg omelets, attending day-care/school, and playing in soil. Common risk factors of salmonellosis such as taking antibiotics 15 days before onset or contact with a patient with diarrhea were not associated.
Comparison of S. Typhimurium and other Salmonella by consumption of different foods (frequency once per week or more) and factors.
| Variables | S. Typhimurium N=148 (%) | Other Salmonella serotypes N=64 (%) | OR 95% CI | p value |
|---|---|---|---|---|
| Age (years)±SD | 12.1±20.6 | 22.8±27.1 | 0.98 (0.97–0.99) | 0.003 |
| Female | 81 (54.7) | 32 (50.0) | 1.21 (0.67–2.17) | 0.527 |
| Country of origin | ||||
| Spain | 135 (91.2) | 59 (92.2) | 0.88 (0.30–2.58) | 0.816 |
| Rumania | 8 (5.4) | 0 (0.0) | 4.98 (0.75–∞) | 0.106 |
| Maghreb | 2 (1.4) | 3 (4.7) | 0.29 (0.05–1.71) | 0.167 |
| Consumption frequency once by week or more | ||||
| Chicken | 145 (98.0) | 60 (93.8) | 3.22 (0.70–14.23) | 0.133 |
| Turkey | 53 (35.8) | 15 (23.4) | 1.87 (0.93–3.66) | 0.079 |
| Veal | 89 (60.1.9) | 38 (59.4) | 1.03 (0.57–1.88) | 0.917 |
| Lamb | 55 (37.2) | 16 (25.0) | 1.77 (0.92–3.42) | 0.087 |
| Pork meat | 120 (81.1) | 40 (62.5) | 2.57 (1.34–4.73) | 0.005 |
| Pork sausages | 94 (63.5) | 30 (46.9) | 1.97 (1.09–3.57) | 0.025 |
| Cold pork meats | 92 (62.2) | 26 (40.6) | 2.64 (1.44–4.82) | 0.002 |
| Dried pork sausages | 59 (39.9) | 12 (18.9) | 2.87 (1.49–5.84) | 0.004 |
| Pork productsa | 138 (93.2) | 48 (75.0) | 4.60 (1.96–10.82) | 0.000 |
| Minced meat | 115 (77.7) | 40 (62.5) | 2.04 (1.11–3.95) | 0.023 |
| Fish | 130 (87.8) | 59 (92.2) | 0.61 (0.22–1.73) | 0.354 |
| Chicken egg mayonnaise | 30 (20.3) | 12 (18.8) | 1.10 (0.52–2.32) | 0.799 |
| Chicken egg omelets | 133 (90.0) | 49 (76.6) | 2.71 (1.24–5.96) | 0.013 |
| Other foods with eggs | 20 (13.5) | 7 (10.9) | 1.27 (0.51–3.18) | 0.606 |
| Drinking unsafe water | 19 (12.8) | 11 (17.2) | 0.61 (0.28–1.37) | 0.406 |
| Raw meat in home kitchen | 121 (81.8) | 58 (90.6) | 0.46 (0.18–1.18) | 0.108 |
| Handling raw meat | 56 (37.8) | 27 (42.2) | 0.83 (0.46–1.51) | 0.552 |
| Restaurant visit 15 days before onset | 59 (39.9) | 17 (26.6) | 1.83 (0.96–3.49) | 0.066 |
| Attend day-care/school | 76 (51.4) | 19 (29.7) | 2.49 (1.34–4.67) | 0.004 |
| Play in soil | 77 (52.0) | 17 (26.6) | 3.0 (1.58–5.70) | 0.001 |
| Play in places with pigeons | 42 (28.4) | 18 (28.1) | 1.01 (0.53–1.94) | 0.970 |
| Animals at home | 77 (52.0) | 34 (53.1) | 0.96 (0.53–1.72) | 0.883 |
| Dog, cat, other mammals | 60 (40.5) | 26 (40.6) | 1.00 (0.55–1.81) | 0.911 |
| Birds | 27 (18.2) | 13 (20.3) | 0.87 (0.41–1.83) | 0.724 |
| Reptiles | 5 (3.4) | 3 (4.7) | 0.71 (0.16–3.07) | 0.648 |
| Take antibiotics 15 days before | 30 (20.3) | 15 (23.4) | 0.83 (0.41–1.68) | 0.605 |
| Contact with diarrhea patients | 19 (12.8) | 7 (10.9) | 1.20 (0.48–3.01) | 0.699 |
OR=odds ratio; CI=confidence interval; SD=standard deviation.
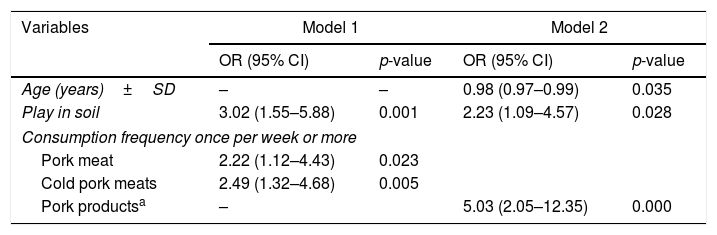
Several logistic regression models were implemented from the associated factors with a significance level of p<0.10. In model 1 (Table 4), consumption of pork meat (OR=2.22 (95% CI 1.12–4.43)), cold pork meats (OR=2.49 (95% CI 1.32–4.68)), and playing in soil (OR=3.02 (95% CI 1.55–5.88)), were associated with S. Typhimurium infection. In model 2, pork products, considered as positive response answer to consumption of pork meat, pork sausages, dried pork sausages and cold pork meats (OR=5.03 (95% CI 2.05–12.35)), and playing in soil (2.23 (95% CI 1.09–4.57)) were associated with S. Typhimurium, adjusted by age (OR=0.98 (95% CI 0.97–0.99)).
Multivariate logistic regression models: S. Typhimurium associated factors.
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years)±SD | – | – | 0.98 (0.97–0.99) | 0.035 |
| Play in soil | 3.02 (1.55–5.88) | 0.001 | 2.23 (1.09–4.57) | 0.028 |
| Consumption frequency once per week or more | ||||
| Pork meat | 2.22 (1.12–4.43) | 0.023 | ||
| Cold pork meats | 2.49 (1.32–4.68) | 0.005 | ||
| Pork productsa | – | 5.03 (2.05–12.35) | 0.000 | |
OR=odds ratio; CI=confidence interval; SD=standard deviation.
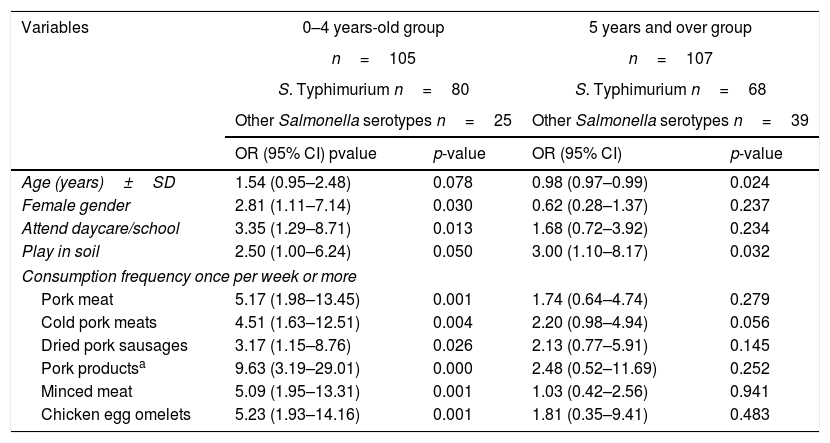
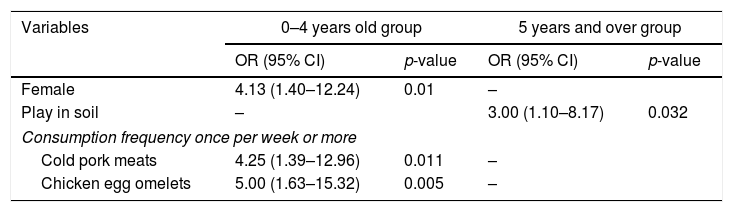
Table 5 shows the 0–4 years old group and 5 years and over group considering the S. Typhimurium and other Salmonella serotypes in each group and the associated factors. In the 0–4 years old group, these factors were female gender, attending day-care/school, and consumptions once per week or more of pork meat, cold pork meats, dried pork sausages, pork products, minced meat, and chicken egg omelets. In the 5 years and over group, the associated factors were age and playing in soil. Table 6 shows the results of the logistic regression. In the 0–4 years old group, the associated factors were consumption of cold pork meats (OR=4.25 (95% CI 1.39–12.96)) and chicken egg omelets (OR=5.00 (95% CI 1.63–15.32)) and female gender (OR=4.13 (95% CI 1.40–12.24)). In addition, the consumption of pork products (OR=12.84 (95% CI 3.76–43.91)) and female gender (OR=4.09 (95% CI 1.34–12.51)) were associated with S. Typhimurium. In the 5 years and over group, only playing in soil (OR=3.00 (95% CI 1.10–8.17)) was associated with S. Typhimurium.
S. Typhimurium associated factors for two age groups: 0–4 years old and 5 years and over.
| Variables | 0–4 years-old group | 5 years and over group | ||
|---|---|---|---|---|
| n=105 | n=107 | |||
| S. Typhimurium n=80 | S. Typhimurium n=68 | |||
| Other Salmonella serotypes n=25 | Other Salmonella serotypes n=39 | |||
| OR (95% CI) pvalue | p-value | OR (95% CI) | p-value | |
| Age (years)±SD | 1.54 (0.95–2.48) | 0.078 | 0.98 (0.97–0.99) | 0.024 |
| Female gender | 2.81 (1.11–7.14) | 0.030 | 0.62 (0.28–1.37) | 0.237 |
| Attend daycare/school | 3.35 (1.29–8.71) | 0.013 | 1.68 (0.72–3.92) | 0.234 |
| Play in soil | 2.50 (1.00–6.24) | 0.050 | 3.00 (1.10–8.17) | 0.032 |
| Consumption frequency once per week or more | ||||
| Pork meat | 5.17 (1.98–13.45) | 0.001 | 1.74 (0.64–4.74) | 0.279 |
| Cold pork meats | 4.51 (1.63–12.51) | 0.004 | 2.20 (0.98–4.94) | 0.056 |
| Dried pork sausages | 3.17 (1.15–8.76) | 0.026 | 2.13 (0.77–5.91) | 0.145 |
| Pork productsa | 9.63 (3.19–29.01) | 0.000 | 2.48 (0.52–11.69) | 0.252 |
| Minced meat | 5.09 (1.95–13.31) | 0.001 | 1.03 (0.42–2.56) | 0.941 |
| Chicken egg omelets | 5.23 (1.93–14.16) | 0.001 | 1.81 (0.35–9.41) | 0.483 |
OR=odds ratio; CI=confidence interval; SD=standard deviation.
Multivariate logistic regression models: S. Typhimurium associated factors by age groups; 0–4 years old and 5 years and over.
| Variables | 0–4 years old group | 5 years and over group | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Female | 4.13 (1.40–12.24) | 0.01 | – | |
| Play in soil | – | 3.00 (1.10–8.17) | 0.032 | |
| Consumption frequency once per week or more | ||||
| Cold pork meats | 4.25 (1.39–12.96) | 0.011 | – | |
| Chicken egg omelets | 5.00 (1.63–15.32) | 0.005 | – | |
OR=odds ratio; CI=confidence interval; SD=standard deviation.
The results of this study suggest that the consumption of pork products and playing in soil were associated with S. Typhimurium infections. However, when two age groups were considered, associated factors of S. Typhimurium infection in the 0–4 years old group were female gender, and consumption of pork products and chicken egg omelets. In the 5 years and over group, playing in soil was the only associated factor in the logistic model. In addition, some differences in clinical symptoms between Salmonella infections case 1 and case 2 groups could be highlighted.
In relation to intestinal symptoms, the patients with S. Typhimurium infections had more severe clinical manifestations than the patients with other Salmonella serotypes. S. Typhimurium produces inflammatory diarrhea with metabolic changes that affect the intestinal microbiota and the type III secretions systems induce the invasion, intracellular survival, and the immune response of the patient.8,9 Young children and old people had symptomatic infections, whereas other adults were asymptomatic or had few gastroenteritis symptoms. Salmonellosis may be considered a disease of children, since the 76% of S. Typhimurium cases were in patients under the age of 10; this percentage was 52% for other Salmonella serotypes. Higher incidence in children and old people has been observed in previous studies.5 Morbidity from positive Salmonella culture account for a small proportion of the total morbidity caused by Salmonella and only potential risk factors associated with these patients could be studied.
Exposure to pork products, and playing in soil were higher in S. Typhimurium patients than other Salmonella patients, suggesting that they could be risk factors for the general population.10 Female gender and consumption of chicken egg omelets were associated with S. Typhimurium in the 0–4 years old group only, and it could be considered more hypothetical. In Castellon, the risk factors of sporadic Salmonella infection in 1–7 years old children were consumption of minced meat and contacts with animals in patients with diarrhea, and in 0–2 years-old children minced meat or poultry in the kitchen were associated with salmonellosis in a case-control study.11,12 In our study, minced meat was a risk factors in the 0–4 years old group but it lost significance in the logistic models. In Salamanca,13 a retrospective study of Salmonella infections found that S. Typhimurium was the most frequent serotype isolated, the patients had 7 years old as median of age, and consumption of pork products is suggested as a risk factor. In a case-control study in China,14S. Typhimurium was more frequent than S. Enteritidis and pork and poultry products were risk factors. In a case-case in Germany, Ziehm and co-authors15 found that S. Typhimurium was associated with consumption of raw ground pork (OR=2.8 95% CI 1.99–3.94) and rodents keeping (OR=1.4 95% CI 1.05–1.89). This study included 867 S. Typhimurium versus 1034 other Salmonella serotypes patients.
Many animals are reservoirs of S. Typhimurium, including swine, cattle, chicken, birds, and reptiles and this serotype is considered ubiquitous as a result exposure is very varied. The pork products associated were pork meat and cold pork meats. The first product is eaten cooked and the second, which includes salami, chorizo, and cured dried pork sausage is eaten raw. In Spain, several studies of Salmonella infections have estimated high prevalence, 43.1% in swine farms,16 31% in pigs17 and 10.8% in processing plants.18 In addition, Salmonella was isolated in 23.7% of raw material for the production of fuet, a fermented pork sausage,19 11.1% of cured dried sausage samples, a ready-to-eat food,20 and 8.3% of pork meat in retail outlets.21 On the other hand, S. Typhimurium infections have been associated with chicken egg consumption in Spain 22 and other countries such as Australia.23 Another risk factor was playing in soil, which may be contaminated by animal feces. These risk factors have been indicated in studies of salmonellosis in children.24 Female gender was associated with S. Typhimurium in the 0–4 years old group but not in the group 5 years and over more; its OR is elevated and it may indicate some gender differences in food preferences and unknown factors.
The study includes a high percentage of Salmonella positive culture cases and the patients were interviewed in acceptable period after the infection onset, which likely reduced interview and recall biases. The high sample size had a sufficient power. Foods consumed were identified as habitual preference.
This study has some limitations. First, there is a potential selection bias arising from the study of patients with Salmonella feces culture, since they may have more serious symptoms or other associated pathologies. Similarly, very young or very old patients could have some differences from the general population. Second, S. Typhimurium group had several phage-types that could differ in their reservoirs or transmission routes; in addition, other Salmonella group included serotypes associated with pork products25 such as S. Derby and S. Rissen, these potential biases could have a deviation toward the null hypotheses. Third, in the case-case design, the common risk factors of Salmonella infections such as antibiotic use, immune-deficiencies, and other pathologies are difficult to detect. Fourth, the questionnaire focuses on foods consumed but other risk factors such as travels could also play some role. Fifth, the 0–4 years old group is homogenous (49.5% of total) and yielded obtain more significant associations than the 5 years and over group (51.5% of total), which had a higher age dispersion. Sixth, the absence of molecular methods to study S. Typhimurium such as pulsed-field gel electrophoresis hampers a more precise epidemiology for finding outbreaks and it may be case that some of what were considered to be sporadic cases were actually from unknown outbreaks.26
Case-case methodology has been used to study salmonellosis outbreaks and presents some advantages over the case-control design. Information bias may be lower but selection bias may increase. The use of case-case design in parallel with epidemiologic surveillance may be indicated; since it reduces the time needed to find appropriate controls for the case, and may allow more adequate measures to stop Salmonella infections.
Increased health education about the consumption of pork products may be required considering that the potential risk of salmonellosis associated with pork products is little known in the Spanish population and consumption of this food is very common; Spain was the second producer of pig meat in Europe in 2014 and the third pork consumer in 2015 with 54.4kg per capita/year.27,28 The risk of salmonellosis by chicken eggs consumption is present and it need more specific actions. In addition, other measures include improvements of food hygiene in the swine industry, a national control program for Salmonella in pigs and pork products29 and the appropriate maintenance of children playgrounds.30 Children are the most affected by STI; pork products and chicken egg omelets consumption and playing in soil are the main associated factors.
Conflict of interestThe authors declare no conflict of interest.
The authors thank all the patients for their cooperation, which has made this study possible.