Acute respiratory tract infections (ARI) are the main cause of morbidity and mortality in children worldwide. The WHO estimated that 920,136 deaths occurred in children less than 5 years old due to pneumonia in 2015.1 In Mexico, ARI were the leading cause of disease in 2016.2 The first multiplex PCR assay for respiratory viruses approved by the U.S FDA was the xTAG-RVPTM (Luminex). In Mexico, the InDRE (National Reference Institute for Diagnosis), uses this method for detection of respiratory viruses. However, the cost is high and a special infrastructure is needed. The AnyplexTM II RV16 was introduced in Mexico in 2013 at a more accessible cost. This kit was approved by the European Community (November 2012), the Canadian Department of Health (July 2012) and the Korea FDA (2013) for the diagnosis of respiratory viruses.3,4 The aim of this study was to compare two multiplex PCR techniques for the detection of respiratory viruses in 310 samples of children with pneumonia.
From March 2010 to August 2013, 310 samples were included from 1404 children from 1 month to 5 years old with clinical or radiological diagnosis of pneumonia admitted at six different hospitals in Mexico. After written informed consent from the parents or guardians, nasal washes were obtained from children using saline solution which was instilled in each nostril using a catheter, aspirated and diluted 1:1 with viral culture media, aliquoted, and stored at −80°C until processing. One aliquot was sent to the InDRE for viral detection by xTAG-RVP and another aliquot was processed simultaneously by the Anyplex II RV16 at the Faculty of Medicine, UNAM. The gold standard was a construct (the same result with both techniques, and those with discrepancies were sequenced). Virus frequencies, sensitivity, specificity, positive and negative predictive values and kappa coefficient were calculated. A total of 271 samples (87.4%) were positive for a virus with either technique; 246 (79.4%) using RV16 and 243 (78.4%) with xTAG-RVP. Overall, the most frequently detected viruses were RSVA, rhinovirus/enterovirus, parainfluenza viruses, and adenovirus. Comparison of both methods showed differences for the detection of RSVA, rhinovirus/enterovirus, metapneumovirus, and adenovirus (Table 1). The RV16 assay detected up to 5 viruses in one sample, one virus in 159 samples (51.3%) and more than one in 87 samples (28.1%); in contrast xTAG-RVP detected up to 3 viruses in one sample, one virus in 193 samples (62.2%) and more than one in 50 samples (16.1%).
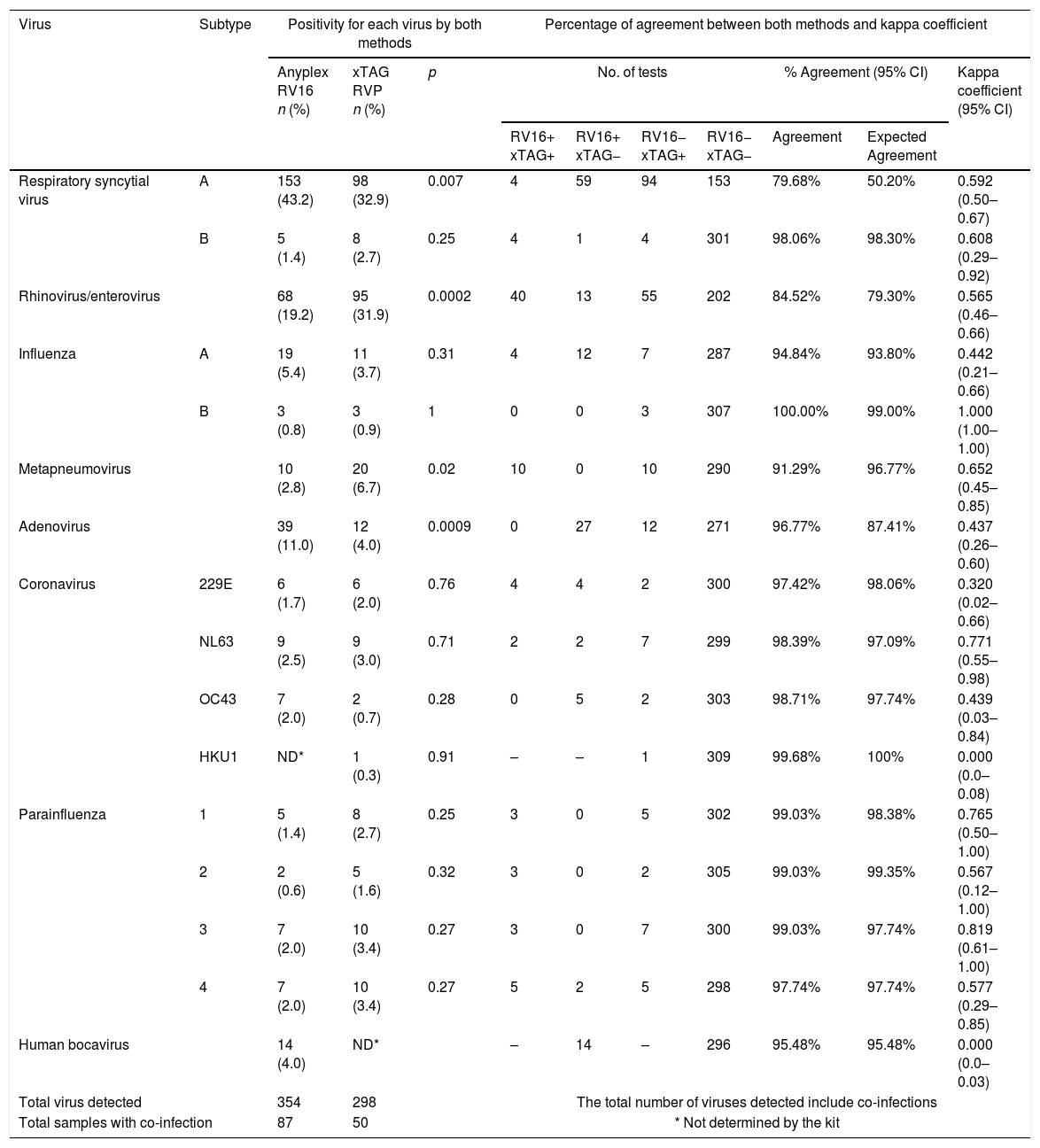
Positivity and agreement for each virus detected by Anyplex RV16 and xTAG RVP in nasal washes of children with pneumonia.
| Virus | Subtype | Positivity for each virus by both methods | Percentage of agreement between both methods and kappa coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anyplex RV16 n (%) | xTAG RVP n (%) | p | No. of tests | % Agreement (95% CI) | Kappa coefficient (95% CI) | ||||||
| RV16+ xTAG+ | RV16+ xTAG− | RV16− xTAG+ | RV16− xTAG− | Agreement | Expected Agreement | ||||||
| Respiratory syncytial virus | A | 153 (43.2) | 98 (32.9) | 0.007 | 4 | 59 | 94 | 153 | 79.68% | 50.20% | 0.592 (0.50–0.67) |
| B | 5 (1.4) | 8 (2.7) | 0.25 | 4 | 1 | 4 | 301 | 98.06% | 98.30% | 0.608 (0.29–0.92) | |
| Rhinovirus/enterovirus | 68 (19.2) | 95 (31.9) | 0.0002 | 40 | 13 | 55 | 202 | 84.52% | 79.30% | 0.565 (0.46–0.66) | |
| Influenza | A | 19 (5.4) | 11 (3.7) | 0.31 | 4 | 12 | 7 | 287 | 94.84% | 93.80% | 0.442 (0.21–0.66) |
| B | 3 (0.8) | 3 (0.9) | 1 | 0 | 0 | 3 | 307 | 100.00% | 99.00% | 1.000 (1.00–1.00) | |
| Metapneumovirus | 10 (2.8) | 20 (6.7) | 0.02 | 10 | 0 | 10 | 290 | 91.29% | 96.77% | 0.652 (0.45–0.85) | |
| Adenovirus | 39 (11.0) | 12 (4.0) | 0.0009 | 0 | 27 | 12 | 271 | 96.77% | 87.41% | 0.437 (0.26–0.60) | |
| Coronavirus | 229E | 6 (1.7) | 6 (2.0) | 0.76 | 4 | 4 | 2 | 300 | 97.42% | 98.06% | 0.320 (0.02–0.66) |
| NL63 | 9 (2.5) | 9 (3.0) | 0.71 | 2 | 2 | 7 | 299 | 98.39% | 97.09% | 0.771 (0.55–0.98) | |
| OC43 | 7 (2.0) | 2 (0.7) | 0.28 | 0 | 5 | 2 | 303 | 98.71% | 97.74% | 0.439 (0.03–0.84) | |
| HKU1 | ND* | 1 (0.3) | 0.91 | – | – | 1 | 309 | 99.68% | 100% | 0.000 (0.0–0.08) | |
| Parainfluenza | 1 | 5 (1.4) | 8 (2.7) | 0.25 | 3 | 0 | 5 | 302 | 99.03% | 98.38% | 0.765 (0.50–1.00) |
| 2 | 2 (0.6) | 5 (1.6) | 0.32 | 3 | 0 | 2 | 305 | 99.03% | 99.35% | 0.567 (0.12–1.00) | |
| 3 | 7 (2.0) | 10 (3.4) | 0.27 | 3 | 0 | 7 | 300 | 99.03% | 97.74% | 0.819 (0.61–1.00) | |
| 4 | 7 (2.0) | 10 (3.4) | 0.27 | 5 | 2 | 5 | 298 | 97.74% | 97.74% | 0.577 (0.29–0.85) | |
| Human bocavirus | 14 (4.0) | ND* | – | 14 | – | 296 | 95.48% | 95.48% | 0.000 (0.0–0.03) | ||
| Total virus detected | 354 | 298 | The total number of viruses detected include co-infections | ||||||||
| Total samples with co-infection | 87 | 50 | * Not determined by the kit | ||||||||
Results obtained by the two techniques showed discrepancies in 63 samples, which were sequenced, and a gold standard construct was made to determine the diagnostic performance of each test. Overall, RV16 and xTAG-RVP had very similar sensitivity (90.4% vs. 89.7%, respectively; p=0.77), specificity (97.4% vs. 100%, respectively; p=0.99), positive predictive value (99.6% vs. 100%, p=0.93) and negative predictive value (59.4% vs. 58.2%, p=0.84). However, for individual viruses some statistically significant differences were observed: RV16 was more sensitive than xTAG-RVP for adenovirus [100% (23/23) vs. 52.2% (12/23); p=0.0001] and RSVA [97.8%, (134/137) vs. 70.8%, (97/137) p<0.001]; in addition, RV16 showed a higher specificity for metapneumovirus detection (100%) compared to xTAG-RVP (97.1%; p=0.004).
The kappa coefficients and percentages of agreement varied widely (Table 1).
This study compares the sensitivity and specificity to detect respiratory viruses by RV16 and xTAG-RVP. Overall, the two tests had similar performance; however, a significant difference in sensitivity was observed for RSVA and adenovirus. Detection of adenovirus represents a significant challenge, because there are 57 serotypes. A lower detection for certain adenovirus serotypes using xTAG-RVP has been reported, with an overall sensitivity of 74.3%.5 Other studies have also shown a lower sensitivity of xTAG-RVP compared with RV16 for adenovirus detection.3–5 One advantage of RV16 compared to xTAG-RVP is the ability to detect human bocavirus. Another advantage of RV16 is the better capacity to detect co-infections and a higher number of viruses in one sample.
In conclusion, RV16 and xTAG-RVP showed similar diagnostic performances. Nevertheless, in scenarios where RSVA, adenovirus, or human bocavirus are important causes of infection RV16 may provide more favorable results. Further evaluations in other clinical settings or sample types would be helpful to guide the selection of the best suited multiplex PCR kit for diagnostic purposes.
FundingThis work was supported in part by grants 182274 (to Wong-Chew, RM) and 69852 (to Santos-Preciado, JI) from the Consejo Nacional de Ciencia y Tecnología (CONACYT) and A.P.G.R. was recipient of a scholarship from CONACYT (grant 182274).
The authors would like to thank Química Valaner and Seegene for the donation of Anyplex RV16 kits, the sequencing of the discordant samples and the financial support to present these results at the IDWeek 2014 Meeting in Philadelphia, PA.
This work was presented as a poster at the ID week 2014 in Philadelphia, Pennsylvania, on October 2014 and the annual meeting of the Asociación Mexicana de Infectología y Microbiología Clínica in Acapulco, Guerrero on May 2014.
Group of study for Pediatric Infectious Diseases: Alejandra P. González-Rodríguez (División de investigación, Facultad de Medicina, Universidad Nacional Autónoma de México), Luis Fernando Perez-González (Hospital Central “Dr. Ignacio Morones Prieto” SLP), Jesús Gaitán-Meza (Nuevo Hospital Civil de Guadalajara "Dr. Juan I. Menchaca"), Alberto Villaseñor-Sierra (Laboratorio de Microbiología Molecular, Centro de Investigación Biomédica de Occidente, IMSS Guadalajara), Gerardo Martínez-Aguilar (Unidad de Investigación Biomédica IMSS Durango, Hospital General de Durango), Oscar A. Newton-Sánchez (Hospital Regional Universitario de los Servicios de Salud de Colima), Verónica Firo-Reyes (Servicio de Pediatría, Hospital General de México “Dr. Eduardo Liceaga”), Carlos Del Río-Almendarez (Hospital Pediátrico de Coyoacan), Celia M. Alpuche-Aranda (Instituto Nacional de Salud Pública, CISEI), Teresa Hernández-Andrade (Instituto de Diagnóstico y Referencia Epidemiológica), Irma López-Martínez (Instituto de Diagnóstico y Referencia Epidemiológica).