To assess the efficacy and safety of hydroxychloroquine (HCQ) compared with no treatment in healthcare workers with mild SARS-CoV-2 infection.
MethodsProspective, non-randomized study. All health professionals with confirmed COVID-19 between April 7 and May 6, 2020, non-requiring initial hospitalization were asked to participate. Patients who accepted treatment were given HCQ for five days (loading dose of 400mg q12h the first day followed by200mg q12h). Control group included patients with contraindications for HCQ or who rejected treatment. Study outcomes were negative conversion and viral dynamics of SARS-CoV-2, symptoms duration and disease progression.
ResultOverall, 142 patients were enrolled: 87 in treatment group and 55 in control group. The median age was 37 years and 75% were female, with few comorbidities. There were no significant differences in time to negative conversion of PCR between both groups. The only significant difference in the probability of negative conversion of PCR was observed at day 21 (18.7%, 95%CI 2.0–35.4). The decrease of SARS-CoV-2 viral load during follow-up was similar in both groups. A non significant reduction in duration of some symptoms in HCQ group was observed. Two patients with HCQ and 4 without treatment developed pneumonia. No patients required admission to the Intensive Care Unit or died. About 50% of patients presented mild side effects of HCQ, mainly diarrhea.
ConclusionsOur study failed to show a substantial benefit of HCQ in viral dynamics and in resolution of clinical symptoms in health care workers with mild COVID-19.
Evaluar la eficacia y seguridad de hidroxicloroquina (HCQ), en comparación con la ausencia de tratamiento en los profesionales sanitarios con infección leve por SARS-CoV-2.
MétodosEstudio prospectivo y no aleatorio. Se solicitó su participación a todos los profesionales sanitarios con diagnóstico confirmado de COVID-19, entre el 7 de abril y el 6 de mayo de 2020, que no requirieron hospitalización inicial. Los pacientes que aceptaron el tratamiento recibieron HCQ durante cinco días (dosis de carga de 400 mg cada 12 h el primer día, y a continuación 200 mg cada 12 h). El grupo control incluyó pacientes con contraindicaciones de HCQ, o que rechazaron el tratamiento. Los resultados del estudio fueron conversión negativa y dinámica viral de SARS-CoV-2, duración de los síntomas y progresión de la enfermedad.
ResultadosEn total se incluyeron 142 pacientes: 87 en el grupo de tratamiento, y 55 en el grupo control. La edad media fue de 37 años, y el 75% fueron mujeres, con pocas comorbilidades. No existieron diferencias significativas en cuanto al tiempo transcurrido hasta la conversión negativa de la PCR entre ambos grupos. La única diferencia significativa en cuanto a la probabilidad de negativización de la PCR se observó el día 21 (18,7%, IC 95% 2-35,4). El descenso de la carga viral de SARS-CoV-2 durante el seguimiento fue similar en ambos grupos. Se observó una reducción no significativa de la duración de algunos síntomas en el grupo HCQ. Dos pacientes con HCQ y cuatro sin tratamiento desarrollaron neumonía. Ningún paciente requirió ingreso en la Unidad de Cuidados Intensivos, ni hubo fallecidos. Cerca del 50% de los pacientes presentó efectos secundarios leves de HCQ, principalmente diarrea.
ConclusionesNuestro estudio no reflejó un beneficio sustancial de HCQ, en cuanto a dinámica viral y resolución de los síntomas clínicos en los profesionales sanitarios con infección leve por COVID-19.
The novel illness caused by the previously unknown betacoronavirus identified as sever acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 and swiftly spread worldwide. The coronavirus disease 2019 (COVID-19) pandemic has stunned the world and health systems. As in other epidemics, health workers are highly affected by the COVID-19 outbreak, but they are essential for their containment and should be carefully protected, both to ensure patient health care and to ensure that they do not transmit the virus themselves. So far, there is no intervention that can reduce the severity and duration of the disease or the period of contagiousness in patients with COVID-19, and in healthcare personnel particularly.
Hydroxychloroquine (HCQ) has been worldwide used as anti-malarial and autoimmune disease drug, with an acceptable safety profile.1,2 It has some antiviral properties as well as immunomodulatory effects,1 a potent in vitro activity against SARS-CoV-2,2–4 and the pharmacokinetic profile seems favorable to treat COVID-19.2,4
Evidence from few small observational and controlled studies on the benefits and harms of using HCQ to treat COVID-19 is weak and conflicting.5 Some studies have reported promising results with HCQ.5–8 Other studies have shown no benefit of HCQ, particularly in patients with severe or critical COVID-19 illness.9–12 Most of these studies included hospitalized patients usually of greater age, with many comorbidities and moderate or severe disease. Moreover, the end-points were mortality reduction and HCQ was usually associated with other antiviral drugs, with or without azithromycin. Therefore, the role of HCQ was difficult to fully elucidate. There is also a growing concern about the risk of prolonged QT intervals when combining HCQ with azithromycin.13–15
Recently, the results of two randomized studies in non-hospitalized patients have been reported.16,17 In both studies, treatment with HCQ did not substantially reduce symptom severity.
The main objective of our study was to assess whether treatment with HCQ alone reduces the time to negative PCR, and the symptoms of healthcare personnel with mild illness by COVID-19, who did not require initial hospitalization.
MethodsStudy setting, design and participantsThis is a prospective, non-randomized, single center study, conducted at the Vall d’Hebron Hospital. This is a 1.100-beds public, university, tertiary hospital in Barcelona, with a total of 6.731 healthcare workers. At the peak of the pandemic, about 650 beds were occupied by COVID-19 patients and by April 30th, 2020, 5.435 patients had been diagnosed of SARS-CoV-2 infection, 2.236 patients with moderate-severe disease had been hospitalized, 335 in critical care unit, and 285 died.
Healthcare workers from Vall d’Hebron Hospital with symptoms compatible with COVID-19 were sent to Occupational Risks Prevention Unit for medical evaluation and to collect pooled nasal and pharyngeal swabs for PCR assay. Patients were eligible for the study if they had PCR confirmed SARS-CoV-2 infection with mild symptoms (eg, fever, acute cough, sore throat, fatigue, headache, muscle pain, sudden gustatory or olfactory loss, but no dyspnea), that allowed confinement at home. Recruitment started on April 7 and ended on May 6, 2020. We included all eligible healthcare professionals diagnosed during this period that gave their consent to participate in the study.
Treatment, procedures and assessmentPatients were divided into two groups: treatment group (patients who accepted the HCQ treatment) and control group (patients with contraindications to HCQ18 or who rejected treatment). Patients in the treatment group were given HCQ at baseline for five days, with a loading dose of 400mg q12h the first day, followed by 200mg q12h the remaining four days, as suggested by Yao et al.4 In both groups symptomatic treatments were given as needed.
The start of follow-up (baseline) for each patient was when they were contacted by a researcher Clinical Pharmacologist and accepted to participate in the study, between 0 and 2 days after PCR confirmed SARS-CoV-2 infection. All patients were followed up from baseline to day 28. Patients were confined at home and received an informed consent. Three telephonic interviews were performed at baseline and at days 14 and 28 to collect demographic and clinical data, and possible side effects of HCQ treatment.
Samples and PCR assayClinical specimens for RT-PCR assay from each patient were obtained initially for diagnosis and according to the study protocol on days 7, 10, 14, 21 and 28 for patients who continued to test positive (±2 days). Viral RNA was extracted from pooled nasal and oropharyngeal swabs and they were processed at the Respiratory Viruses Unit of the Microbiology Department. The diagnosis of COVID-19 was initially performed by two commercial RT-PCR-based assays, Allplex™ 2019-nCoV (Seegene, Korea) and Cobas® SARS-CoV-2 (Roche Diagnostics, USA) tests. In addition, to evaluate viral dynamics, an in-house RT-PCR assay using the primer/probe set targeting the nucleocapsid protein (N1) and the human RNase P (housekeeping gene) from the CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel was also carried out. High-moderate viral load were defined as those with a cycle threshold (Ct) value for N1<35. The Ct values of the viral target were normalized to a housekeeping gene based on the ΔCt method (Ctsample−Cthousekeeping gene) in order to minimize the variations due to the non-standardized collection of a heterogenous specimen.
The primary outcome for this trial was the proportion of patients in which the first control PCR at day 7 (±2) was negative. Secondary objectives included: time to negative conversion of PCR, proportion of patients and probability of negative PCR conversion at 14, 21 and 28 days, dynamics of ΔCt, duration of clinical symptoms, and progression to pneumonia, hospitalization or death.
Statistical analysisConsidering an approximate ratio of 3:2 of patients accepting to be treated with HCQ vs. control patients, 75–84 treated patients and 49–53 control patients achieve a minimum power of 80% to detect a difference of 25% in the main variable between both groups. For continuous variables, description of the sample has been made with the median and quartiles, and comparisons between groups with the Wilcoxon-Mann-Whitney test. For categorical variables, frequency and percentage for description, and chi-square test for comparisons. In addition, 95% confidence interval has also been calculated for the percentage differences. To compare the time to the negative status of the PCR tests, a Kaplan–Meier analysis was used with the log-rank test. Probabilities of reaching the outcome at predefined time points (7, 10, 14, 21, and 28 days) were also compared. Finally, a polynomial regression has been adjusted to explore the evolution of ΔCt as a function of time for each group. The p value for statistical significance has been set at 0.05 and all tests have been considered bilateral. The analyses have been carried out with the SAS® 9.4 (SAS Institute Inc., Cary, NC, USA) and R 4.0.2 (R Core Team, http://www.R-project.org) programs.
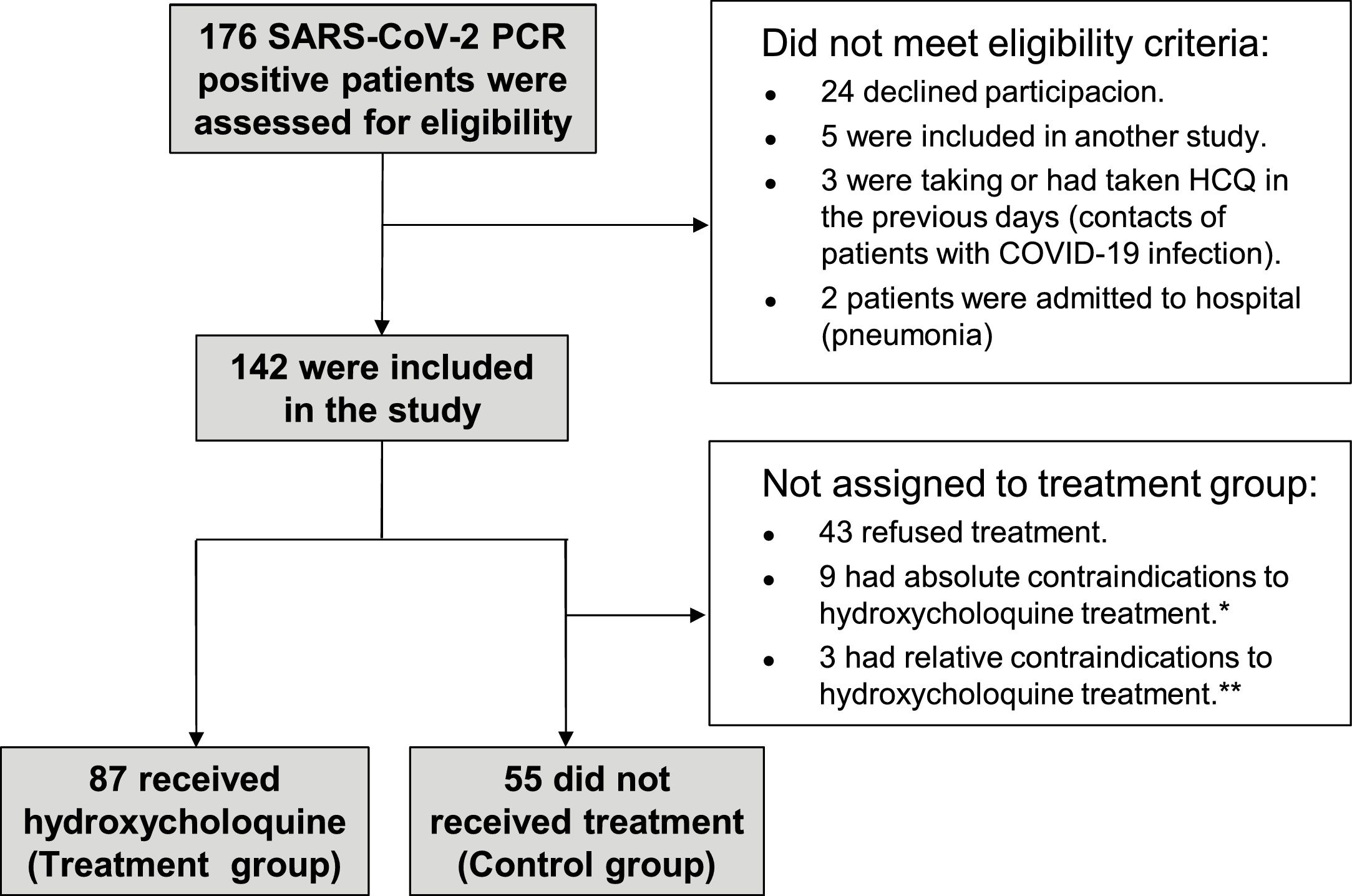
ResultsPatients and baseline characteristicsOf 176 healthcare workers with PCR confirmed SARS-CoV-2 infection from 7 April to 6 May of 2020, 34 were not included in the study, mainly because they refused study participation. Characteristics of patients not included in the study were similar to those of included patients. Of the remaining 142, 87 received HCQ treatment, and 55 were included in the control group, being the main reason treatment refusal (Fig. 1).
Flow diagram of trial participants. *Absolute contraindications included: 2 patients with history of hypersensibility to hydroxichloroquine, 3 patients with retinopathy, 3 patients with psoriasis and 1 miastenia gravis.**Relative contraindications included: 2 patients with right blockage of bundle branch and 1 atopic dermatitis.
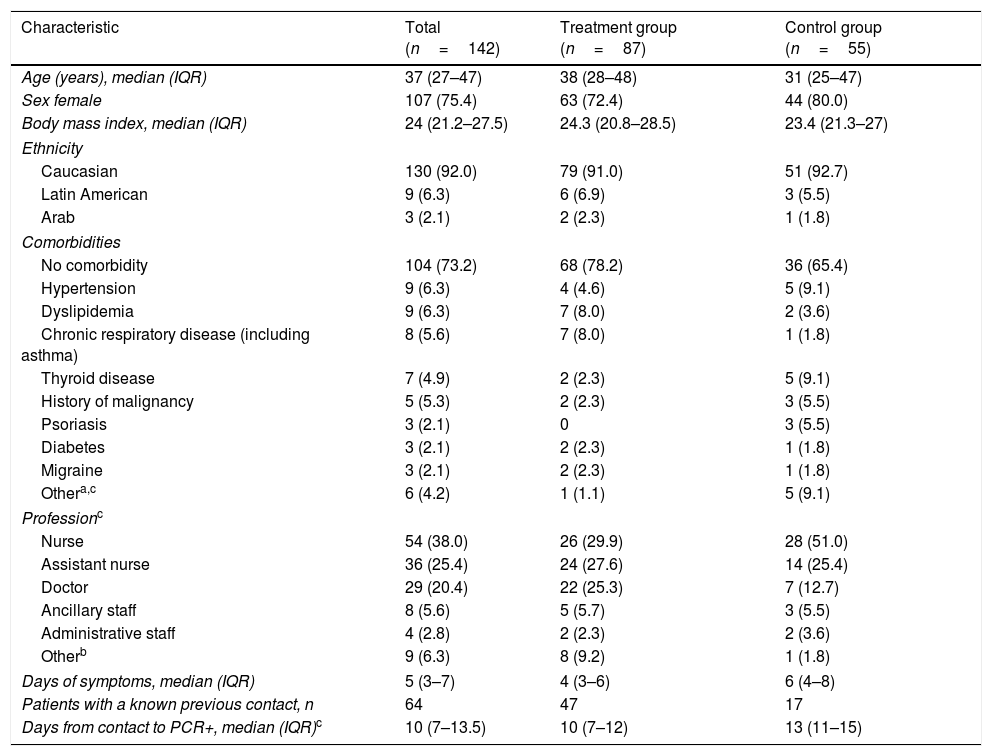
Table 1 shows the baseline demographic and clinical characteristics of patients in both groups. The median age of patients was 37 years and 75% were female. The median body mass index was 24. Patients in both groups had few comorbidities; being arterial hypertension, dyslipidemia and asthma the most common. There were more medical doctors in the treatment group (25%) and more nurses in the control group (52%). The median interval between symptoms onset and inclusion to the study was 5 days.
Study population characteristics at baseline by treatment group.
| Characteristic | Total (n=142) | Treatment group (n=87) | Control group (n=55) |
|---|---|---|---|
| Age (years), median (IQR) | 37 (27–47) | 38 (28–48) | 31 (25–47) |
| Sex female | 107 (75.4) | 63 (72.4) | 44 (80.0) |
| Body mass index, median (IQR) | 24 (21.2–27.5) | 24.3 (20.8–28.5) | 23.4 (21.3–27) |
| Ethnicity | |||
| Caucasian | 130 (92.0) | 79 (91.0) | 51 (92.7) |
| Latin American | 9 (6.3) | 6 (6.9) | 3 (5.5) |
| Arab | 3 (2.1) | 2 (2.3) | 1 (1.8) |
| Comorbidities | |||
| No comorbidity | 104 (73.2) | 68 (78.2) | 36 (65.4) |
| Hypertension | 9 (6.3) | 4 (4.6) | 5 (9.1) |
| Dyslipidemia | 9 (6.3) | 7 (8.0) | 2 (3.6) |
| Chronic respiratory disease (including asthma) | 8 (5.6) | 7 (8.0) | 1 (1.8) |
| Thyroid disease | 7 (4.9) | 2 (2.3) | 5 (9.1) |
| History of malignancy | 5 (5.3) | 2 (2.3) | 3 (5.5) |
| Psoriasis | 3 (2.1) | 0 | 3 (5.5) |
| Diabetes | 3 (2.1) | 2 (2.3) | 1 (1.8) |
| Migraine | 3 (2.1) | 2 (2.3) | 1 (1.8) |
| Othera,c | 6 (4.2) | 1 (1.1) | 5 (9.1) |
| Professionc | |||
| Nurse | 54 (38.0) | 26 (29.9) | 28 (51.0) |
| Assistant nurse | 36 (25.4) | 24 (27.6) | 14 (25.4) |
| Doctor | 29 (20.4) | 22 (25.3) | 7 (12.7) |
| Ancillary staff | 8 (5.6) | 5 (5.7) | 3 (5.5) |
| Administrative staff | 4 (2.8) | 2 (2.3) | 2 (3.6) |
| Otherb | 9 (6.3) | 8 (9.2) | 1 (1.8) |
| Days of symptoms, median (IQR) | 5 (3–7) | 4 (3–6) | 6 (4–8) |
| Patients with a known previous contact, n | 64 | 47 | 17 |
| Days from contact to PCR+, median (IQR)c | 10 (7–13.5) | 10 (7–12) | 13 (11–15) |
Data are n (%) unless otherwise stated.
Other comorbidities group included: 1 atopic dermatitis in the treatment group and 1 myasthenia gravis, 1 rheumatic disease, 1 glaucoma, 1 vitreous detachment, and 1 celiac disease in the control group.
The percentage of patients with a negative viral load for SARS-CoV-2 in their nasopharyngeal swap samples was numerically higher in the treatment group than in control group in the different sampling PCRs: day 7, 25.6% vs. 14.3% (primary outcome); day 10, 43.5% vs. 36%; day 15, 61.2% vs. 47.1%, day 22, 80% vs 71.2%. However, no differences were statistically significant (Table S1, Supplementary data).
Most patients had a high/moderate SARS-CoV-2 viral load (PCR N1 Ct<35) at baseline, with an early viral clearance in both groups. Differences between both groups were lower than 10% and not significant (Table S2, Supplementary data).
Kaplan-Meier curves of time to negative conversion of SARS-CoV-2 PCR from nasopharyngeal swaps showed no significant differences between both groups (Fig. 2). The median time of negative PCR conversion was 15 days (95% CI, 11–18) in treatment group and 22 days (95%CI, 14–24) in the control group. The maximum and only significant difference in the probability of negative conversion was 18.7% (95%CI 2.0 to 35.4%, p=0.034) at day 21 (Table S3, Supplementary data).
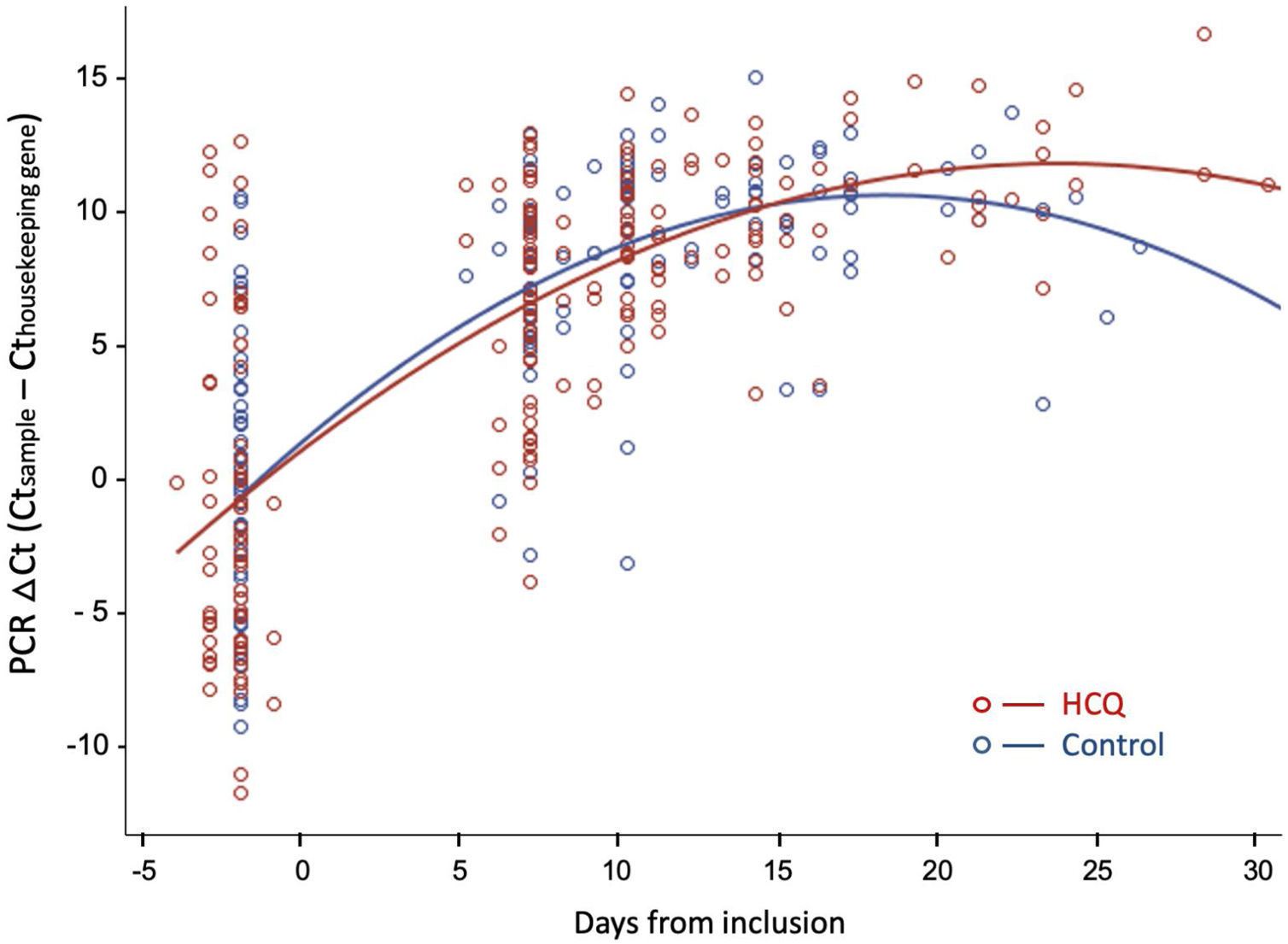
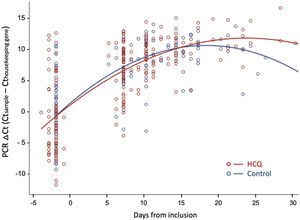
Fig. 3 shows the evolution of normalized viral load values during follow-up in both groups, fitted by second-degree polynomial curves. The ΔCt (Ct sample−Ct reference) was similar in both groups at baseline and during follow-up.
Viral dynamics: Normalized viral loads of the nasopharyngeal swab samples at different times of disease onset from patients treated with HCQ and patients without treatment, estimated with the Δ Ct method (CtN1 Cthuman RNase P). Note that higher viral loads are inversely related to Ct values.
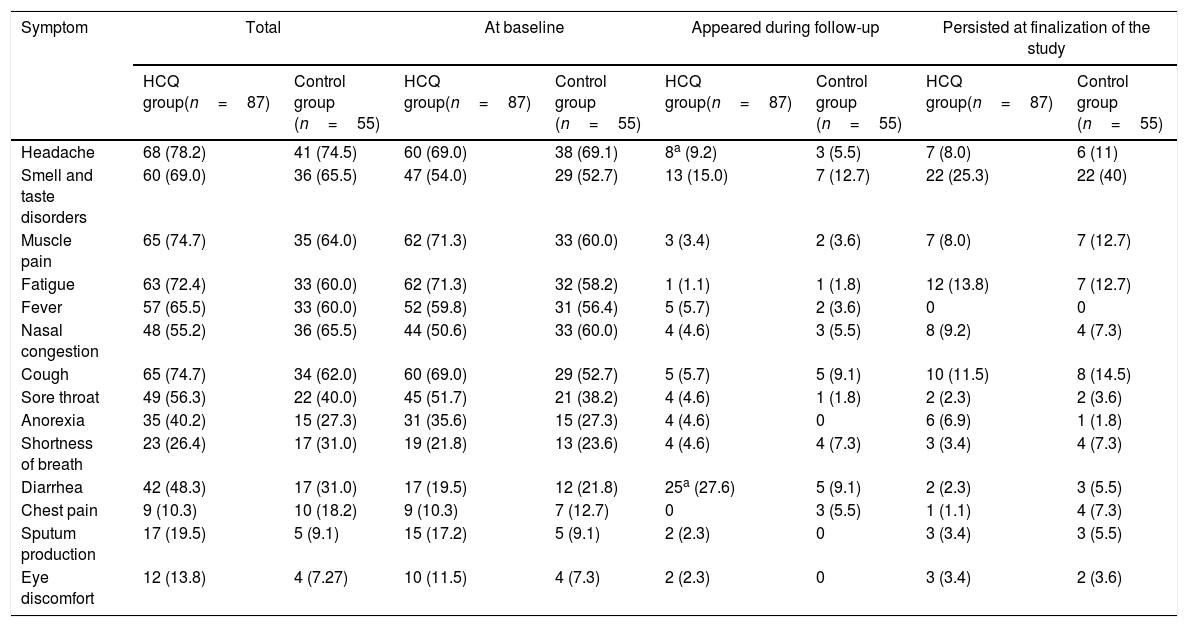
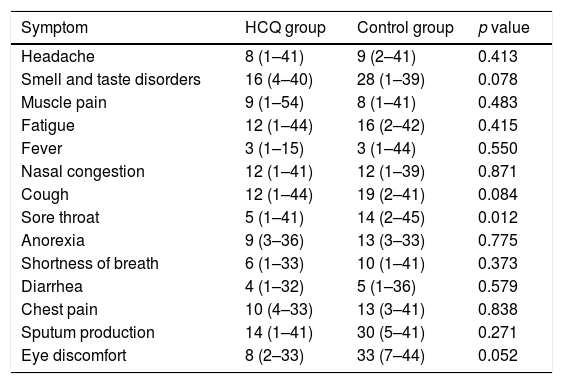
The most common symptoms were headache, muscle pain, fatigue, smell and taste disorders, fever, nasal congestion and cough. Smell and taste disorders and diarrhea appeared in a higher proportion of patients during follow-up and smell and taste disorders, fatigue and cough persisted in more patients at finalization of the study period (Table 2). Symptoms with a longer duration were smell and taste disorders, fatigue, nasal congestion and cough (Table 3). The duration of sore throat was significantly longer in patients without treatment in comparison to the treatment group, but the number of patients with this symptom was low in both groups. The duration of smell and taste disorders and cough was also longer in patients without HCQ treatment, but the difference was not significant.
Summary of symptoms presents at baseline, that appeared during follow-up and that persisted at finalization of the study, in patients with COVID-19 in treatment group or control group.
| Symptom | Total | At baseline | Appeared during follow-up | Persisted at finalization of the study | ||||
|---|---|---|---|---|---|---|---|---|
| HCQ group(n=87) | Control group (n=55) | HCQ group(n=87) | Control group (n=55) | HCQ group(n=87) | Control group (n=55) | HCQ group(n=87) | Control group (n=55) | |
| Headache | 68 (78.2) | 41 (74.5) | 60 (69.0) | 38 (69.1) | 8a (9.2) | 3 (5.5) | 7 (8.0) | 6 (11) |
| Smell and taste disorders | 60 (69.0) | 36 (65.5) | 47 (54.0) | 29 (52.7) | 13 (15.0) | 7 (12.7) | 22 (25.3) | 22 (40) |
| Muscle pain | 65 (74.7) | 35 (64.0) | 62 (71.3) | 33 (60.0) | 3 (3.4) | 2 (3.6) | 7 (8.0) | 7 (12.7) |
| Fatigue | 63 (72.4) | 33 (60.0) | 62 (71.3) | 32 (58.2) | 1 (1.1) | 1 (1.8) | 12 (13.8) | 7 (12.7) |
| Fever | 57 (65.5) | 33 (60.0) | 52 (59.8) | 31 (56.4) | 5 (5.7) | 2 (3.6) | 0 | 0 |
| Nasal congestion | 48 (55.2) | 36 (65.5) | 44 (50.6) | 33 (60.0) | 4 (4.6) | 3 (5.5) | 8 (9.2) | 4 (7.3) |
| Cough | 65 (74.7) | 34 (62.0) | 60 (69.0) | 29 (52.7) | 5 (5.7) | 5 (9.1) | 10 (11.5) | 8 (14.5) |
| Sore throat | 49 (56.3) | 22 (40.0) | 45 (51.7) | 21 (38.2) | 4 (4.6) | 1 (1.8) | 2 (2.3) | 2 (3.6) |
| Anorexia | 35 (40.2) | 15 (27.3) | 31 (35.6) | 15 (27.3) | 4 (4.6) | 0 | 6 (6.9) | 1 (1.8) |
| Shortness of breath | 23 (26.4) | 17 (31.0) | 19 (21.8) | 13 (23.6) | 4 (4.6) | 4 (7.3) | 3 (3.4) | 4 (7.3) |
| Diarrhea | 42 (48.3) | 17 (31.0) | 17 (19.5) | 12 (21.8) | 25a (27.6) | 5 (9.1) | 2 (2.3) | 3 (5.5) |
| Chest pain | 9 (10.3) | 10 (18.2) | 9 (10.3) | 7 (12.7) | 0 | 3 (5.5) | 1 (1.1) | 4 (7.3) |
| Sputum production | 17 (19.5) | 5 (9.1) | 15 (17.2) | 5 (9.1) | 2 (2.3) | 0 | 3 (3.4) | 3 (5.5) |
| Eye discomfort | 12 (13.8) | 4 (7.27) | 10 (11.5) | 4 (7.3) | 2 (2.3) | 0 | 3 (3.4) | 2 (3.6) |
Data are n (%)
Symptom durationa in patients with COVID-19 in treatment group or control group.
| Symptom | HCQ group | Control group | p value |
|---|---|---|---|
| Headache | 8 (1–41) | 9 (2–41) | 0.413 |
| Smell and taste disorders | 16 (4–40) | 28 (1–39) | 0.078 |
| Muscle pain | 9 (1–54) | 8 (1–41) | 0.483 |
| Fatigue | 12 (1–44) | 16 (2–42) | 0.415 |
| Fever | 3 (1–15) | 3 (1–44) | 0.550 |
| Nasal congestion | 12 (1–41) | 12 (1–39) | 0.871 |
| Cough | 12 (1–44) | 19 (2–41) | 0.084 |
| Sore throat | 5 (1–41) | 14 (2–45) | 0.012 |
| Anorexia | 9 (3–36) | 13 (3–33) | 0.775 |
| Shortness of breath | 6 (1–33) | 10 (1–41) | 0.373 |
| Diarrhea | 4 (1–32) | 5 (1–36) | 0.579 |
| Chest pain | 10 (4–33) | 13 (3–41) | 0.838 |
| Sputum production | 14 (1–41) | 30 (5–41) | 0.271 |
| Eye discomfort | 8 (2–33) | 33 (7–44) | 0.052 |
Data are median days (minimum–maximum).
In our study, 2 patients (2.3%) in the HCQ group and 4 patients (7.3%) in the control group developed pneumonia (OR 0.30, 95%CI, 0.05–1.70). No patients required admission to the Intensive Care Unit or died.
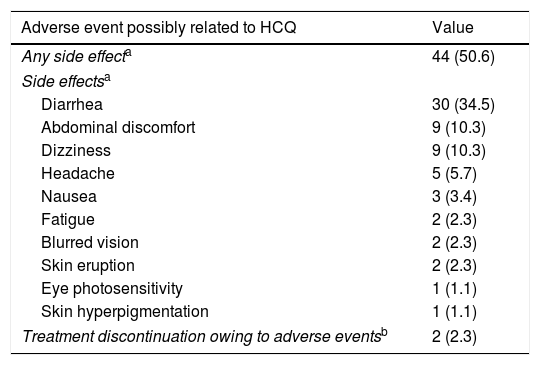
Side effects with HCQ treatmentDuring follow-up, 44/87 (50.6%) patients in the treatment group reported 64 possible side effects associated with HCQ treatment (Table 4). Diarrhea was the most common side effect, reported in 30 (34.5%) patients, in 25 of them diarrhea appeared after starting HCQ and in 5 diarrhea worsened. It was mostly mild, but in two cases led to treatment withdrawal. Side effects had not resolved at the end of the study period in only three patients; including a case of skin hyperpigmentation and another case of ocular photosensitivity.
Summary of side effects in patients treated with hydroxychloroquine.
| Adverse event possibly related to HCQ | Value |
|---|---|
| Any side effecta | 44 (50.6) |
| Side effectsa | |
| Diarrhea | 30 (34.5) |
| Abdominal discomfort | 9 (10.3) |
| Dizziness | 9 (10.3) |
| Headache | 5 (5.7) |
| Nausea | 3 (3.4) |
| Fatigue | 2 (2.3) |
| Blurred vision | 2 (2.3) |
| Skin eruption | 2 (2.3) |
| Eye photosensitivity | 1 (1.1) |
| Skin hyperpigmentation | 1 (1.1) |
| Treatment discontinuation owing to adverse eventsb | 2 (2.3) |
Data are n (%)
In our study, a comprehensive analysis of the evolution of nasopharyngeal SARS-CoV-2 viral load and follow-up of symptoms until 28 days postinclusion in the study was performed. Five day treatment with HCQ in healthcare professionals with mild SARS-CoV-2 infection did not significantly reduce time to negative PCR-test. Differences in the percentages of patients with negative PCR at days 7, 10, 14 and 28 were not statistically significant. The only marginally significant difference in the probability of negative conversion of PCR was observed at day 21, but it was a secondary variable of the study. Furthermore, there were no differences in the evolution of the amount of viral load during follow-up in both groups. In addition, duration of some symptoms tended to be shorter in those patients treated with HCQ, but the difference was not statistically significant. A considerable proportion (approximately 50%) of the patients treated with HCQ had side effects possibly associated to its use, mainly diarrhea, although mild in most cases.
Patients included in our study were young, predominantly female and with few comorbidities, similar to those of the trials by Skipper et al.16 and by Mitjà et al.17 As a difference, all of our patients were healthcare professionals. The percentages of nurses (∼60%) and medical doctors (∼20%) are in accordance with the distribution of COVID-19 infected healthcare professional during the pandemia in our hospital. A higher percentage of medical doctors received treatment with HCQ, mainly because a higher percentage of nurses rejected treatment with HCQ.
We found few differences in the evolution of patients in both groups. These negative results have been confirmed in the study by Mitjà et al.17 They did not find differences in the mean reduction of viral RNA load in the nasopharyngeal swaps at days 3 and 7 after initiation of treatment. Nevertheless, in our study, a longer follow-up of the evolution of nasopharyngeal viral loads was obtained. The maximum difference in the percentage of patients with a negative PCR-test between groups was observed at 21 days.
Headache, smell and taste disorders, muscle pain, cough and fatigue were in general the most frequently reported symptoms during the study, and smell and taste disorders and diarrhea were those that appeared with a higher frequency during follow-up. However, in most cases HCQ could be the cause of diarrhea when it appeared after treatment initiation. Smell and taste disorders, fatigue and cough persisted longer during the study period and although the median time duration of smell and taste disorders and cough tended to be shorter with HCQ treatment, the differences between groups were not statistically significant. In the study by Skipper et al.,16 no differences in the evolution of symptoms between the treatment and placebo groups were found either. In this study, change in overall symptoms severity was the main variable, but information on symptom severity was only obtained until day 14. In our study, very few patients were diagnosed with pneumonia during follow-up in both groups and none required admission at the intensive care unit or died. In the study by Mitjà et al.,17 the clinical outcome of risk of hospitalization was similar in both arm groups and no patient required mechanical ventilation no deaths were reported during the study.
Several reasons have to be taken into consideration when explaining why a drug that has shown promising data in vitro against SARS-CoV2 has obtained disappointing results in clinical studies. Firstly, it has been suggested that the disparity between laboratory and clinical data may be due at least in part to the complex pharmacokinetics of 4-aminoquinolines, making it difficult to extrapolate concentrations in culture media to doses in humans.19 Secondly, the variability of the doses and duration of HCQ treatment between studies has been high, and the effect of different loading and maintenance doses or the duration of treatments on clinical outcomes has not been properly addressed in the studies.20 However, negative results in several studies using different posology have already been published, and therefore, this could mean that in fact dose variability between studies is not the key. Finally, the Ct cut-off level used to report PCR-test as positive or negative has also been variable between studies.6,9 In our laboratory, the routine cut-off level >40 is reported as PCR-negative, but when a cut-off of 35 was tested for this study the proportion of negative results was higher. However, differences between both groups were not significant for both cut-offs.
An argument often used to justify the use of HCQ was its safety and the broad experience in its use to treat certain rheumatic diseases.2,19 However, concerns arose when in several studies a prolongation of QT interval and in some cases potential lethal arrhythmias were documented, especially in patients treated with concomitant azithromycin.12–14 In our study more than half of the patients treated with HCQ had side effects, however the severity was mostly mild, being diarrhea and gastrointestinal discomfort the most common. No electrocardiogram monitoring was performed during the study, but no patients reported possible cardiac side effects. It should be noted that our patients were younger and with less comorbidity than those included in the studies where the concerns on the cardiac side effects of HCQ in combination with azithromycin emerged. In addition, in our study patients with contraindications to HCQ were excluded from treatment. In the studies of Mitjà et al.17 and Skipper et al.,16 with similar populations and the same or slightly higher doses of HCQ than in our study, the proportion of participants with adverse events was even higher than in our study, but also with only mild side effects.
Our study has several limitations. Firstly, it was not a randomized study therefore we cannot rule out a possible influence of factors that could drive the preferences of patients when they decided to accept or reject treatment. Secondly, the study was powered to detect a difference of 25% in the percentage of negative PCR-test at 7 days post-inclusion. It is possible that the expected difference has been oversized and that in fact the real difference is lower. However, our results are in accordance with those of some recently published clinical trials in patients with similar characteristics. One of the main strengths of our study is that all professional healthcare workers with SARS-CoV2 infection during the study period were detected and asked for participation. In addition, all the included patients had a confirmed COVID19 infection by a positive PCR-test in the nasopharyngeal swap. Furthermore, a comprehensive follow-up of participants was carried out during the study in order to fulfill the scheduled PCR-tests and obtain the information on symptom evolution and side effects to treatment. Finally, information on the evolution of viral RNA load was provided at several time-points during the study and for a longer period of time than in other recently published studies with also more extensive information on the evolution of symptoms.
In conclusion, our study failed to show a substantial benefit of HCQ in viral dynamics and in resolution of clinical symptoms. Treatment with HCQ was associated with a numerically higher percentage of negative PCR-tests at some points during follow-up in comparison with the non-treatment group, and a shorter duration of some symptoms was also seen with HCQ, but differences were only marginally significant and not clinically relevant. Adverse events possibly associated with HCQ appeared in about 50% of patients, but were mild. These negative results are in accordance with those reported in recently published randomized clinical trials and, therefore do not support the use of HCQ in patients with mild COVID19 infection.
EthicsThe study was conducted according to international ethical recommendations. In accordance with the national directives in relation to post-authorization studies, the study was approved by the Ethics Committee of Clinical Investigation of the Vall d’Hebron University Hospital (Institutional Review Board – 03/04/2020) and registered on the European Union electronic Register of Post-Authorization Studies (EU PAS Register Number EUPAS34570).
FundingThis trial had no financial support. Laboratorios Rubió contributed to the study with the required doses of hydroxychloroquine (Dolquine). Laboratorios Rubió had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflict of interestAll authors declare no conflict of interest.
We thank all the healthcare worker patients participating in the study.