Hemophagocytic lymphohistiocytosis (HLH) is an aggressive and life-threatening syndrome characterized by excessive immune activation. We analyzed the presentation, diagnosis and prognosis of our cohort of HLH-Leishmania cases.
MethodsWe studied HLH cases in patients over 14 years of age in the province of Granada (Spain), from January 2008 to November 2019.
ResultsIn this study, Leishmania was the predominant trigger of adult HLH in our region. There were no differences in the clinical-analytical presentation between HLH triggered by Leishmania and those initiated by a different cause. RT-PCR was the best tool to identify Leishmania as the trigger of HLH, given that the other microbiological tests showed low sensitivity to detect the parasite in our HLH-Leishmania cases.
ConclusionA comprehensive search for Leishmania is mandatory in HLH cases. Based on our findings, we propose that RT-PCR for Leishmania in bone marrow samples must be included in HLH differential diagnostic protocols.
La linfohistiocitosis hemofagocítica (LHH) es un síndrome agresivo y potencialmente mortal caracterizado por una activación inmune excesiva. Analizamos la presentación, el diagnóstico y el pronóstico de nuestra cohorte de casos LHH-Leishmania.
MétodosEstudiamos los casos de LHH en pacientes mayores de 14 años en la provincia de Granada (España) desde enero de 2008 hasta noviembre de 2019.
ResultadosEn este estudio, la Leishmania fue el desencadenante principal de la LHH en adultos en nuestra región. No hubo diferencias en la presentación clínico-analítica entre la LHH desencadenada por Leishmania y las iniciadas por otra causa. La PCR en tiempo real fue la mejor herramienta para identificar la Leishmania como el desencadenante de LHH, dado que las otras pruebas microbiológicas mostraron baja sensibilidad para detectar el parásito en nuestros casos de LHH-Leishmania.
ConclusiónUna búsqueda exhaustiva de la Leishmania es obligatoria en los casos de LHH. Considerando nuestros hallazgos, proponemos que la PCR en tiempo real de Leishmania en médula ósea se incluya en los protocolos de diagnóstico diferencial de LHH.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and devastating heterogeneous disorder in which defects in natural killer (NK) and T-cell granule-mediated cytotoxic function produce an uncontrolled inflammatory immune response and cytokine storm that lead to tissue damage and organ failure. Based on the HLH-2004 trial,1 the diagnosis is performed when ≥5 of the following criteria are fulfilled: fever, splenomegaly, cytopenia, hypertriglyceridemia and/or hypofibrinogenemia, tissular hemophagocytosis phenomena, low NK-cell activity, hyperferritinemia and elevated sCD25. The number of reported HLH cases, especially in the adult population, has increased dramatically in the last years, probably due to the rise in the identification of the syndrome.2
The initial causative agent in most HLH cases is an inflammatory insult. As regards this, even if the predominant causes differ per country and age group, infectious diseases and neoplasms are the most common triggers worldwide.3
Visceral leishmaniasis (VL), also known as kala-azar, is a systemic infectious disease affecting the reticuloendothelial system caused by protozoa of the genus Leishmania, which are transmitted by the bite of infected phlebotomine sandflies. VL is globally spread and is endemic in more than 60 countries, with about 100,000 new cases each year.4 In Spain Leishmania is endemic, especially in Mediterranean areas, and has an incidence of 0.76 cases/100,000 inhabitants per year. The province of Granada is on the national average (0.49–0.95 cases/100,000 inhabitants per year).5
An association between VL and HLH is well-described in the literature, especially in childhood.6–8 Indeed, the Leishmania parasite is the most common protozoan zoonotic trigger of HLH.3,9 In spite of this, the association between both conditions is uncommon as Leishmania is the suggested trigger of HLH in only 0.77–2.1% of the cases.3,10 In the present work, we begin by providing a general description of HLH cases to proceed then to analyze the presentation, diagnosis and prognosis of the HLH-Leishmania entity in our cohort.
MethodsWe collected HLH cases of patients over 14 years of age as diagnosed following the HLH-2004 criteria1 in the two tertiary hospitals (Hospital Universitario Virgen de las Nieves and Hospital Universitario San Cecilio) in the province of Granada, in southern Spain. The timeframe of our search begins in January 2008 and finishes in November 2019.
We retrospectively collected demographic characteristics (sex, age), clinical features (presence of immunosuppression, highest measured fever and hepatosplenomegaly), laboratory findings (hemoglobin, leucocytes and platelet count, triglyceride, ferritin, lactate dehydrogenase [LDH], liver function tests, creatinine, C-reactive protein [CRP] and fibrinogen), evidence of the presence of hemophagocytosis phenomena, suggested trigger, treatment administered and outcome. The demographic, clinical, laboratory and pathological findings were obtained from the medical records of the day in which the diagnosis of HLH was made.
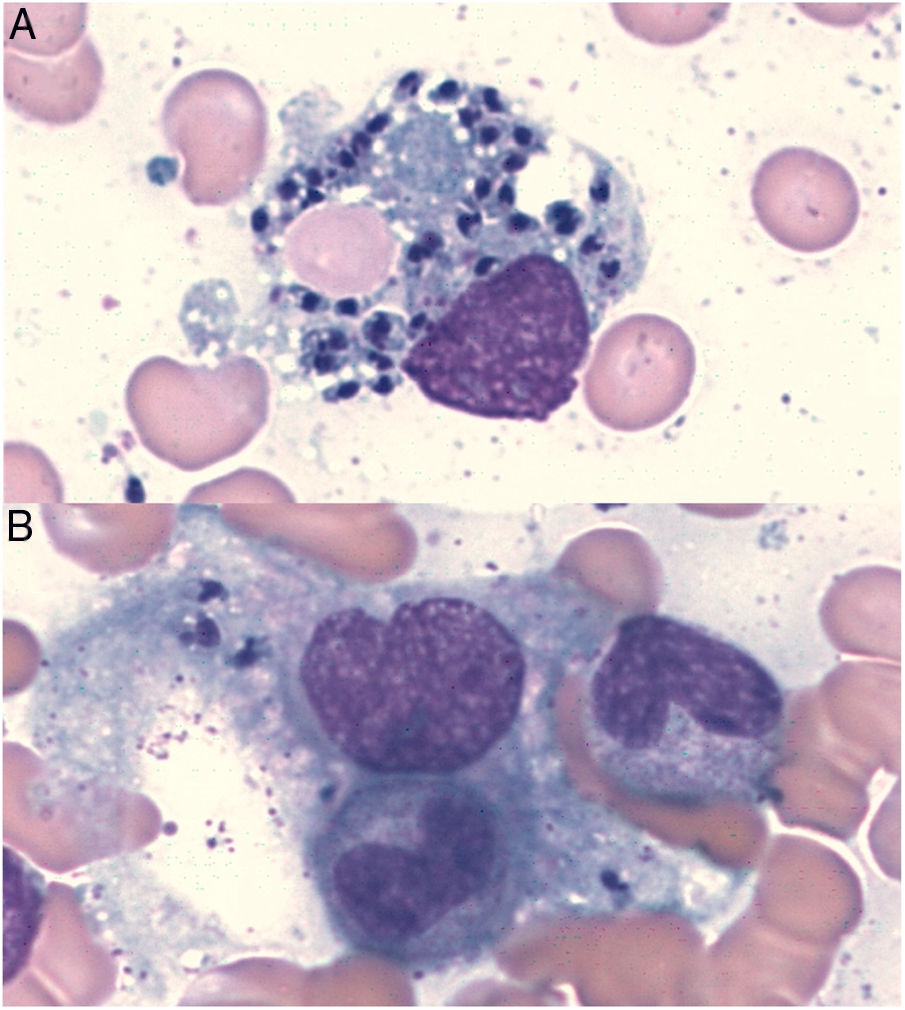
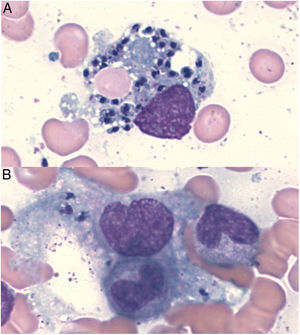
In Leishmania-triggered HLH cases we also collected microbiological findings that allowed the diagnosis, such as parasite visualization (Fig. 1), leishmanial antigen in urine (latex agglutination test, KAtex), molecular detection of DNA (Real-Time PCR [RT-PCR]) in bone marrow and spleen (in those cases in which the splenectomy had been performed) and parasite isolation by in vitro culture (Novy-MacNeal-Nicolle medium).
We first provide a basic descriptive analysis of the data and then we proceed with the statistical analysis. Categorical variables are analyzed using Pearson's Chi-squared test, while continuous variables are compared with two-tailed, independent-samples Student's t-test or Mann–Whitney U test when there was a deviation from normality in the data. We set the significance level to α<0.05.
ResultsThirty patients were diagnosed with HLH in the timeframe analyzed. Infectious diseases were the most common triggers -13 cases (43.3%: 6 Leishmania, 4 CMV, 1 EBV, 1 HIV and 1 Brucella) – followed by oncohematological triggers – 6 cases (20%: 2 T and 2 B lymphomas, 1 acute lymphoblastic leukaemia and 1 colorectal cancer)-, autoimmune triggers – 6 cases (20%: 4 systemic lupus erythematosus, 1 inflammatory bowel disease and 1 antiphospholipid syndrome) – and idiopathic causes – 5 cases (16.6%)-.
There were no statistically significant differences in the characteristics or in the clinical-analytical presentation between HLH triggered by Leishmania and those cases initiated by a different cause (see Supplementary material).
The clinical and analytical characteristics at presentation, the results of the microbiological diagnosis tests and the follow-up of HLH-Leishmania patients can be found in Table 1.
Clinical and analytical characteristics at presentation, results of the microbiological diagnostic tests and follow-up of HLH-Leishmania patients.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
| Sex | F | M | M | M | M | F |
| Age (years) | 27 | 72 | 46 | 43 | 67 | 47 |
| Immunosupression | − | − | +(HIV) | − | − | − |
| Fever (°C) | 38.5 | 38.7 | 39.5 | 40.8 | 38.8 | 38.5 |
| Hepatomegaly | + | − | + | + | + | + |
| Splenomegaly | + | + | + | + | + | + |
| Lymphadenopathy | − | − | + | − | + | − |
| Cytopenia | ||||||
| Hb (g/dL) | 6.4 | 11.1 | 6.7 | 8.9 | 7.5 | 7.7 |
| Neutr. (/μl) | 1640 | 1570 | 250 | 700 | 1510 | 910 |
| Plt. (/μl) | 87,000 | 47,000 | 13,000 | 73,000 | 62,000 | 20,000 |
| Triglycerides (mg/dL) | 232 | 223 | 164 | 411 | 216 | 459 |
| Fibrinogen (mg/dL) | NA | NA | NA | NA | 263 | 93 |
| Ferritin (ng/mL) | 1790 | 14,135 | 2799 | 3581 | 5528 | 11,995 |
| Hemophagocytosis | +(BM and Spleen) | +(BM) | +(BM) | +(Spleen) | +(BM) | +(BM) |
| Creatinine (mg/dL) | 0.9 | 1.3 | 4 | 0.9 | 2.38 | 0.48 |
| Total Bilirrubin (mg/dL) | 1.1 | 1.26 | 19.9 | 0.44 | 15.3 | 2.63 |
| ALT/AST (IU/L) | 74/68 | 337/184 | 16/18 | 143/67 | 143/293 | 16/53 |
| LDH (IU/L) | 946 | 3250 | 384 | 847 | 573 | 135 |
| CRP (mg/L) | 84 | 157 | 255 | 142 | 253 | 201 |
| Time from the onset of symptoms to diagnosis (days) | ||||||
| HLH | 43 | 11 | 64 | 59 | 35 | 64 |
| VL | 657 | 7 | 42 | 63 | 35 | 62 |
| Microbiological diagnostic tests | ||||||
| Parasite visualization | −+(at relapse) | + | + | − | − | + |
| RT-PCR | +(in spleen retrospectively)+(in BM at relapse) | +(in BM) | +(in BM) | +(in BM and spleen) | +(in BM) | +(in BM) |
| Urine Ag | −+(at relapse) | + | − | − | + | + |
| Culture (NNN) | −+(at relapse) | + | − | NA | NA | − |
| Therapy | CS, IVIG, CycA, VP-16At relapse: Tocilizumab and LAmB | CS, CycA, IVIG, LAmB | CS, IGIV, LAmB | CS, LAmB | CS, IVIG, LAmB | CS, IVIG, LAmB |
| Outcome | Alive | Death(non HLH or VL related) | Death(HLH and VL related) | Alive | Alive | Alive |
| Relapse | ||||||
| HLH | + | − | − | − | − | − |
| VL | − | − | − | − | − | − |
| Median follow-up (months) | 136 | 66 | 2 | 77 | 42 | 8 |
ALT/AST, Alanine Aminotransferase/Aspartate Aminotransferase; BM, Bone marrow; CRP, C-reactive protein; CS, Corticosteroids; CycA, Cyclosporine; F, Female; Hb, Hemoglobin; HIV, Human immunodeficiency virus; HLH, Hemophagocytic lymphohistiocytosis; IVIG, Intravenous immunoglobulin; LAmB, Liposomal amphotericin; LDH, Lactate deshydrogenase; M, Male; NA, No available; Neutr, Neutrophil; NNN, Novy-MacNeal-Nicolle medium; Plt, Platelets; RT-PCR, Real-time PCR; VL, Visceral leishmaniasis; VP-16, Etoposide.
In 2 of the cases, a splenectomy was performed due to suspicion of a splenic lymphoproliferative disorder at the beginning of process. The HLH-2004 treatment protocol1 was initiated in one of them because there were no analytical findings suggestive of Leishmania (negative visualization and culture in the bone marrow and negative antigen in urine [RT-PCR was not performed]). Interestingly, this patient had a deteriorating clinical course with HLH relapse despite the multiple immunosuppressant treatments administered. We then obtained a new bone marrow sample where we had a direct visualization of the parasite and positive culture results. Accordingly, a diagnosis of disseminated Leishmania was made and the corresponding treatment was initiated. In order to determine if the parasite was present before the immunosuppressant treatment, we performed an RT-PCR for Leishmania in the previous splenectomy sample (taken at the time of HLH diagnosis), obtaining a positive result.
All the six Leishmania patients were treated with liposomal amphotericin B (5mg/kg on days 1, 5 and 10 and monthly for 3 additional doses) and methylprednisolone (0.5–1mg/kg/daily). One patient with HIV developed multiorgan failure and died 4 days after the HLH diagnosis. Importantly, the mortality rate of Leishmania patients (16.6%) was lower than the rate of patients without Leishmania (45.8%), although this difference did not reach statistical significance (X12=1.7; p=0.19).
ConclusionsThe overlap in the clinical and analytical presentation between HLH and VL and the fact that HLH triggered by Leishmania is indistinguishable from that triggered by a different agent, make the diagnosis of this association a considerable challenge. Leishmania, far from being an infrequent cause of HLH, is the predominant trigger of adult HLH in our region, being the causative agent in 20% of all the HLH cases. This percentage is considerably higher than those reported in the previous studies,3,10 which may be attributable to three factors: first, the study was done in an endemic area; second, the preexisting clinical suspicion of the HLH-Leishmania association; and third, the use of molecular tests to detect the parasite. In our opinion, due to diagnostic challenges, this association may be underestimated with fatal consequences in endemic areas.
VL, caused by Leishmania infantum (the only endemic species in the Iberian Peninsula) and Leishmania donovani, is the single form of leishmaniasis related with HLH. Although the precise mechanisms of Leishmania-triggered HLH onset remain unknown, it seems that the Th1/Th2 disbalance and the cytokine storm produced by the abnormal T-lymphocyte response induced by the parasite may result in uncontrolled activation of the mononuclear phagocyte system leading to HLH.11,12
The identification of the exact trigger in HLH is mandatory. The precise treatment of the causing agent will likely result in the elimination of the stimulus that initially caused the abnormal immune activation.2,3 Consequently, it can be argued that those therapies specifically aimed at the treatment of the triggering agent will be essential in patients with secondary HLH7,8,13 making other aggressive treatments such as chemotherapy-based protocols or allogeneic hematopoietic stem cell unnecessary. These considerations are especially important in those cases of HLH associated with infections where severe immunosuppression without an adequate antimicrobial agent could be devastating. Consequently, the prognosis seems to be better in those HLH cases where Leishmania is identified and treated.7,8,10 However, in our experience, in cases where the cytokine release syndrome has been identified, the addition of low-dose corticosteroids and immunoglobulins to the treatment of the trigger could be beneficial to cease the cytokine storm. In the deceased patient in our series, the diagnosis of HLH trigged by Leishmania was performed when the disease had already progressed. This emphasizes the need of the early diagnosis of this association.
Excluding the presence of Leishmania should be mandatory in every HLH patient with all possible tools, given that a multiple test approach for the diagnosis of Leishmania is currently recommended.4,14 However, it is crucial to keep in mind that the visualization of the parasite in the bone marrow smear, the detection of leishmanial antigen in urine and the in vitro culture have low sensitivity to identify patients with combined HLH and Leishmania, probably because of the paucimicrobial nature of the disorder.6–8,12 In our study, DNA-based assays seem to be the best tool to identify Leishmania as the trigger of HLH, being positive in all of our cases. Thus, leishmanial RT-PCR in bone marrow should be included in HLH protocols. In future studies, other diagnostic methods like RT-PCR in peripheral blood, serological tests (recombinant antigen rK39, direct agglutination tests, enzyme-linked immunosorbent assays or indirect fluorescent antibody tests) or cell-mediated immune response tests should be explored to ascertain their usefulness in identifying Leishmania as HLH trigger.
Furthermore, the importance of the association between HLH and Leishmania could be bidirectional. Hypertriglyceridemia has been identified as a marker of severity in VL.15 This could identify those VL cases where the inflammation is more severe and where the HLH may be underrecognized.16 Consequently, the early detection of these cases by the clinician is vital to evaluate the need for complementary immunosuppressant therapy.
In light of our findings, we suggest that an active and intensive search of Leishmania is required in every case of HLH in endemic areas. Moreover, because of the current migratory movements and the ensuing rise of Leishmania-HLH cases in non-endemic areas,10,13 the proposed Leishmania search strategy should be applied worldwide.
AuthorshipJB, LMM, JLC, ADG, MJ and JHQ: analyzed and interpreted the data, revised and approved the final manuscript. JB, LMM and JHQ: contributed manuscript writing.
FundingThe author(s) received no specific funding for this work.
Conflict of interestThe authors do not have potential conflicts of interest.