Lady Windermere syndrome (LWS) is a pulmonary disease caused by Mycobacterium avium complex (MAC). The objective of this study is to ascertain its frequency and characteristics in the northern area of the autonomous community of Castile and León.
MethodsA retrospective study of patients with MAC isolates in respiratory samples from five public hospitals in the autonomous community over a 6-year period, following the ATS/IDSA criteria. The MAC strains were identified by GenoType Mycobacterium reverse hybridisation probes or PCR-RFLP analysis of the hsp65 gene.
ResultsOf 183 cases of MAC identified, only five women (2.7%) aged 68.8±10.7 years met LWS criteria. In three cases, MAC was isolated jointly and intermittently with other pathogens. Only one patient was treated according to ATS/IDSA criteria.
DiscussionLWS remains underestimated, with affected patients representing a significant burden on healthcare resources over long periods of time. As a result, greater microbiological and therapeutic knowledge of the syndrome is needed.
El síndrome de Lady Windermere (SLW) es una patología pulmonar causada por Mycobacterium avium complex (MAC). El objetivo es conocer su frecuencia y sus características en el área norte de la comunidad de Castilla y León.
MétodosEstudio retrospectivo de pacientes con aislamientos de MAC en muestras respiratorias de cinco hospitales públicos de la comunidad a lo largo de seis años, siguiendo criterios de la ATS/IDSA. Las cepas de MAC se identificaron por sondas de hibridación inversa Genotype Mycobacterium o PCR-RFLP del gen hsp65.
ResultadosDe 183 casos de MAC identificados, únicamente 5 (2,7%) mujeres de 68,8±10,7 años cumplían criterios de SLW. En tres casos se aisló MAC conjunta e intermitentemente con otros patógenos. Solo un paciente se trató siguiendo criterios de la ATS/IDSA.
DiscusiónEl SLW permanece infraestimado, y al ser los afectados muy demandantes de recursos sanitarios durante largos periodos, es necesario un mayor conocimiento microbiológico y terapéutico.
Infection with non-tuberculous mycobacteria (NTM) is an emerging disease in both immunosuppressed and immunocompetent patients.1,2 Mycobacteria belonging to the Mycobacterium avium complex (MAC) are among the most frequently isolated NTM in patients with cystic fibrosis and AIDS. They generally cause chronic lung disease with variable, non-specific symptoms. One pattern caused by MAC is Lady Windermere syndrome (LWS).3 This was first reported in 1989 by Prince et al.4 and subsequently named by Reich and Johnson5 based on the maxim “Ladies don’t spit” from the play Lady Windermere's Fan by Oscar Wilde. It mainly affects immunocompetent elderly women with no history of smoking or lung disease. It essentially damages the middle lobe and the lingula.3 One theory with respect to predisposition to this disease is based on the fact that voluntary cough inhibition hinders elimination of secretions and facilitates inflammation, appearance of bronchiectasis and ultimately infection with MAC. However, this theory has never been proved and few reported cases reflect this fact.1 Another hypothesis that explains the marked predisposition to impairment of the middle lobe is based on an interaction between the lung/chest anatomy and personal habits.6 Although the literature features few case series,4,5,7–11 the disease is recognised in an official document from the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) establishing criteria for its diagnosis and treatment.3
The objective of this study was to determine the frequency of LWS in the northern area of the Autonomous Community of Castile and León in the last 6 years, as well as the clinical, microbiological and radiological characteristics of patients with LWS.
Material and methodsThis was a retrospective study of all cases of patients with MAC isolates in respiratory samples recorded in the microbiology information systems of five public hospitals in Castile and León (Complejo Asistencial Universitario de León, Complejo Asistencial Universitario de Burgos, Complejo Asistencial Universitario de Zamora, Hospital del Bierzo Ponferrada and Hospital Clínico Universitario de Valladolid) between January 2010 and December 2016. Patients who presented with mycobacteriosis according to the ATS/IDSA criteria (positive culture of at least two sputum series or one bronchoalveolar lavage/bronchial aspirate) fitting the pattern of LWS were selected.3 Clinical and epidemiological data as well as information on treatment and clinical course in each case were collected by reviewing each patient's medical history.
Bacterial and mycobacterial culture was performed on respiratory samples according to normal procedures.3 At three of the healthcare centres, isolated MAC strains were identified using GenoType Mycobacterium CM reverse hybridisation probes (Hain Lifescience, Germany). At the other two, said strains were sent to the Spanish National Mycobacteria Centre (CNM) in Majadahonda, Spain, to be identified by means of PCR-RFLP of the hsp65 gene using the BstEII and HaeIII restriction enzymes.
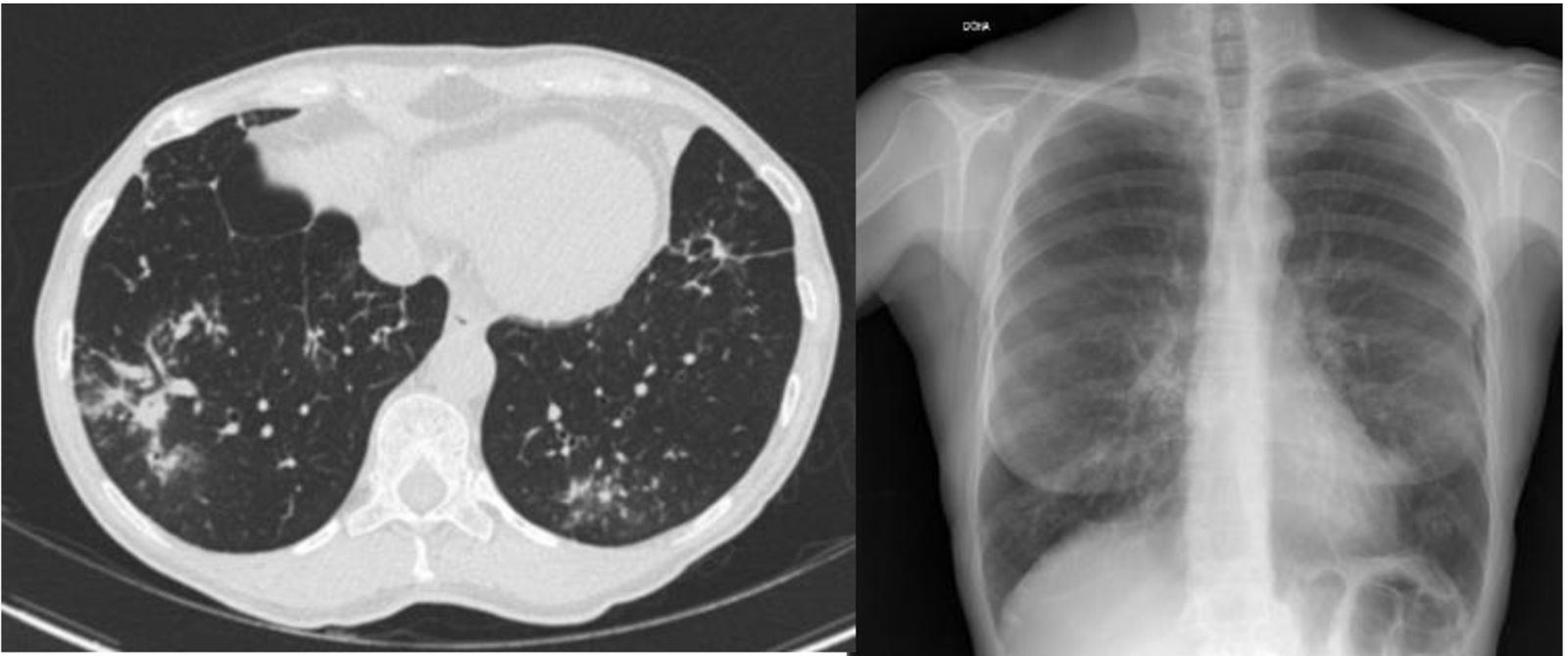
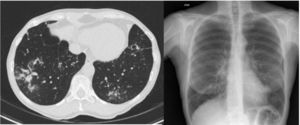
ResultsIn total, 183 cases of patients with MAC were identified in 6 years of follow-up. Their distribution by health area was as follows: 56 in León, 38 in Burgos, 34 in El Bierzo, 32 in Valladolid and 23 in Zamora. Just five (2.7%) met criteria for LWS: four recorded in León and one recorded in Burgos. These patients’ main clinical characteristics, radiological (Fig. 1) and microbiological characteristics and therapeutic actions are shown in Table 1.
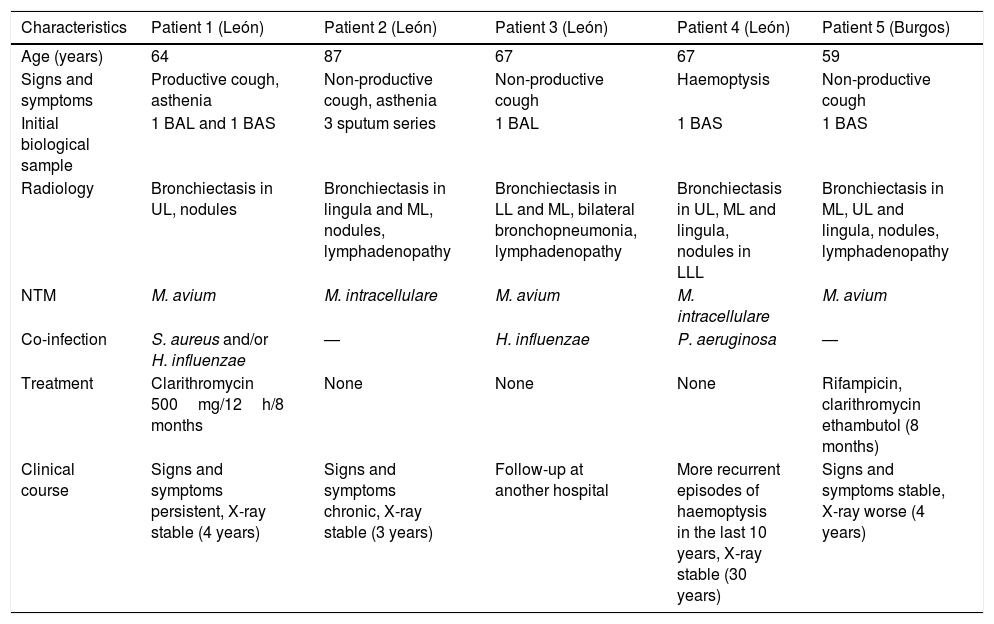
Main characteristics in terms of diagnosis, signs and symptoms, and clinical course of patients with LWS.
| Characteristics | Patient 1 (León) | Patient 2 (León) | Patient 3 (León) | Patient 4 (León) | Patient 5 (Burgos) |
|---|---|---|---|---|---|
| Age (years) | 64 | 87 | 67 | 67 | 59 |
| Signs and symptoms | Productive cough, asthenia | Non-productive cough, asthenia | Non-productive cough | Haemoptysis | Non-productive cough |
| Initial biological sample | 1 BAL and 1 BAS | 3 sputum series | 1 BAL | 1 BAS | 1 BAS |
| Radiology | Bronchiectasis in UL, nodules | Bronchiectasis in lingula and ML, nodules, lymphadenopathy | Bronchiectasis in LL and ML, bilateral bronchopneumonia, lymphadenopathy | Bronchiectasis in UL, ML and lingula, nodules in LLL | Bronchiectasis in ML, UL and lingula, nodules, lymphadenopathy |
| NTM | M. avium | M. intracellulare | M. avium | M. intracellulare | M. avium |
| Co-infection | S. aureus and/or H. influenzae | — | H. influenzae | P. aeruginosa | — |
| Treatment | Clarithromycin 500mg/12h/8 months | None | None | None | Rifampicin, clarithromycin ethambutol (8 months) |
| Clinical course | Signs and symptoms persistent, X-ray stable (4 years) | Signs and symptoms chronic, X-ray stable (3 years) | Follow-up at another hospital | More recurrent episodes of haemoptysis in the last 10 years, X-ray stable (30 years) | Signs and symptoms stable, X-ray worse (4 years) |
BAL, bronchoalveolar lavage; BAS, bronchial aspirate; LL, lower lobe; LLL, left lower lobe; ML, middle lobe; UL, upper lobe.
All were women with a mean age of 68.8±10.7 years (range 59–87 years). Three of these women were notably naturally lean, and one of these three women had scoliosis as a predisposing factor.1,3,7–11 In all cases, microbiological follow-up subsequent to diagnosis consisted of culture of 2–3 sputum series, sent every 4–6 months over a clinical course of years. These always showed growth of the initially isolated mycobacterium. In three cases, they also showed concomitant or intermittent growth of other pathogens (Table 1).
DiscussionIn the last 20 years, the incidence of pulmonary infection with MAC has been increasing2; however, LWS remains an uncommon and probably underestimated disease due to the complexity of the differential diagnosis of a colonisation versus an infection as well as the low sensitivity of sputum cultures (most often, the samples initially sent for diagnosis are sputum samples).8,9
Given the limited number of cases in our study, it could not be established that there was a real epidemiological difference in terms of LWS between the different areas studied. This may have been due to the frequency of distribution of this NTM in the different areas, the collection of clinical/radiological information or the selection of BAL and/or BAS as diagnostic samples.
A review of the Medline, Medical Key, Google Scholar and WoS databases revealed only seven reported case series.4,5,7–11 Therefore, we believe that the five cases presented are a notable contribution to the knowledge of this disease. In our series, all patients were women, as in most reported cases of LWS,4,5,7–11 although anecdotally at least one case has been published of a man who met the established inclusion criteria.12 To date, no clear reason has been unearthed for this gender predilection. A wide range of hypotheses have been put forth, including the role of sex hormones and mediators such as leptin and adiponectin in the release of certain cytokines (TGF-β) and the expression of macrophage receptors (Fcγ).9,13
M. avium and Mycobacterium intracellulare are not usually differentiated in clinical practice. The pathogenic differences between the two species are debated in the literature. Some authors have insisted that there are no pathogenic differences between them14; others maintain that M. intracellulare is associated with a poorer clinical course and response to treatment.10 Our study yielded no conclusive findings in this regard, although the patient with the poorest clinical course (recurrent episodes of haemoptysis for 30 years) was infected with M. intracellulare and intermittently presented co-infection with Pseudomonas aeruginosa in monitoring studies. This is a common combination throughout the literature. P. aeruginosa has been linked to greater pulmonary dysfunction. It is difficult to determine whether this decline was due to the type of isolates obtained or the long clinical course of that case compared to all the others. A total of three of the cases reported presented co-infections at different points in their disease. The pathogens isolated were Haemophilus influenzae and Staphylococcus aureus.3,6
Infection with MAC develops gradually and may take months or years to manifest, generally in a mild, non-specific form. Few patients develop a severe form.10 As a result, it is difficult to decide on starting a therapeutic regimen. The risk–benefit ratio must be weighed up in each case in view of the therapeutic regimens and toxicities of the different options. At present, if there is no radiological worsening (progression of bronchiectasis or appearance of further cavities), close monitoring is done. The start of treatment consists of triple therapy for a year in which the use of a macrolide in monotherapy is avoided to prevent the development of resistance. In this series of cases, just one patient decided to start the therapeutic treatment proposed by the ATS/IDSA3; after 8 months of treatment, this patient experienced clinical stability with radiological worsening. One patient started a macrolide in monotherapy due to intolerance to all the other antibiotics and remained radiologically stable but had marked signs and symptoms. This happened even though the patient did not develop resistance and this drug is the most important one in the therapeutic regimen. Administering it in monotherapy in cases of established infection proves inadequate, whereas administering it as prophylaxis may be considered.2,3 Other studies of LWS have found the proportion of patients receiving treatment to be 20–50% (United States, Canada and South Korea) and have noted high rates of intolerance to antibiotic therapy and therapeutic failure due to persistent or recurrent symptoms.2,7–9
Our series contributed useful information to the knowledge of this disease which is so uncommon in our setting. It had the limitations inherent to retrospective studies and the small number of patients collected. LWS affects patients who require large amounts of healthcare resources for long periods of time. Therefore, greater knowledge of the strains of MAC that this disease produces must be acquired by means of their genotyping in order to develop new strategies that are more efficient and effective than the current strategies.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Blanco-Conde S, Nebreda-Mayoral T, Labayru-Echeverría C, Brezmes-Valdivieso MF, López-Medrano R, Nogueira-González B. Síndrome de Lady Windermere en Castilla y León. Enferm Infecc Microbiol Clin. 2018;36:644–647.