The objective of this report is to describe the clinical pathway for early treatment of patients with acute SARS-CoV-2 infection and to evaluate the first results of its implementation.
MethodsThis is a descriptive and retrospective study of the implementation of a clinical pathway of treatment in outpatients (January 1 to June 30 2022). Clinical pathway: detection and referral systems from Primary Care, Emergency services, hospital specialities and an automated detection system; clinical evaluation and treatment administration in the COVID-19 day-hospital and subsequent clinical follow-up. Explanatory variables: demographics, comorbidity, vaccination status, referral pathways and treatment administration. Outcome variables: hospitalization and death with 30 days, grade 2−3 toxicity related to treatment.
ResultsTreatment was administered to 262 patients (53,4% women, median age 60 years). The treatment indication criteria were immunosupression (68,3%), and the combination of age, vaccination status and comorbidity in the rest 47,3% of the patients s received remdesivir, 35,9% nirmatrelvir/ritonavir, 13,4% sotrovimab and 2,4% combined treatment with a median of 4 days after symptom onset. Hospital admission was required for 6,1% of the patients, 3,8% related to progression COVID-19. No patient died. Toxicity grade 2−3 toxicity was reported in 18,7%, 89,8% dysgeusia and metallic tasted related nirmatrelvir/ritonavir. Seven patients discontinued treatment due to toxicity.
ConclusionThe creation and implementation of a clinical pathway for non-hospitalized patients with SARS-CoV-2 infection is effective and it allows early accessibility and equity of currently available treatments.
El objetivo del manuscrito es describir la vía clínica de tratamiento precoz de pacientes con infección aguda por SARS-CoV-2 y evaluar los primeros resultados de su implementación
MétodosEstudio descriptivo y retrospectivo de la implementación de una vía clínica de tratamiento en pacientes no-hospitalizados, (1 de enero al 30 de junio 2022). Elaboración de vía clínica: sistemas detección y derivación desde Atención Primaria, servicio de Urgencias, especialidades médicas y sistema de detección automatizada; evaluación clínica y administración de tratamiento en hospital de día COVID-19, y seguimiento clínico posterior. Variables explicativas: demográficas, comorbilidad, estado vacunal, vías de derivación y administración de tratamiento. Variables de resultado: hospitalización y muerte a los 30 días, toxicidad grado 2−3 relacionada con el tratamiento.
ResultadosSe administró tratamiento a 262 pacientes (53,4% mujeres, mediana de edad 60 años). Criterio indicación tratamiento: inmunosupresión (68,3%) y la combinación de edad, estado vacunal y comorbilidad en el resto. El 47,3% de los pacientes recibieron remdesivir, el 35,9% nirmatrelvir/ritonavir, el 13,4% sotrovimab y el 2,4% tratamiento combinado, con una mediana de 4 días tras inicio de síntomas. El 6.1% de los pacientes precisó ingreso hospitalario, 3,8% por progresión COVID-19. Ningún paciente falleció. El 18,7% presentaron toxicidad grado 2−3: 89,8% disgeusia y sabor metálico relacionado con nirmatrelvir/ritonavir. Siete pacientes interrumpieron tratamiento por toxicidad.
ConclusiónLa creación e implementación de una vía clínica para pacientes no-hospitalizados con infección por SARS-CoV-2 es efectiva y permite la accesibilidad temprana y la equidad de los tratamientos actualmente disponibles.
At the start of the SARS-CoV-2 pandemic, therapeutic efforts focused on hospitalised patients with moderate-to-severe coronavirus disease 2019. Subsequently, after identifying the populations with the highest risk of severe disease and mortality, clinical trials have been carried out on the early treatment of the infection, with the aim of preventing its progression to severe disease. During 2021, a new field was opened in the treatment of acute infection by SARS-CoV-2 with the development of monoclonal antibodies directed against viral proteins (bamlanivimab/etesevimab,1 casirivimab/imdevimab2 and sotrovimab3), as well as antivirals (remdesivir administered in a 3-day regimen4, nirmatrelvir/ritonavir5 and molnupiravir6).
Given the limited availability of these treatments, regulatory agencies (such as the US National Institutes of Health or the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Drugs and Medical Devices]) have established clinical conditions that prioritize higher-risk populations in which these treatments would be indicated.7
In Spain, before the start of the new epidemic wave triggered by the Omicron variant (December 2021–January 2022), only remdesivir was available as a treatment for non-hospitalised patients with mild infection. Subsequently, there was a gradual introduction of the new drugs (regulated by the AEMPS), with the incorporation of sotrovimab (version 2, 20 January 2022) and nirmatrelvir/ritonavir or molnupiravir (version 3, 24 March 2022). In these last two versions, the treatment indication criteria have been expanded (version 4 and 5, 30 May and 2 August 2022, respectively).8
Within the PROA team (team for optimising the treatment of infections) at our centre, we set out in January 2022 to optimise the treatment of patients with acute SARS-CoV-2 infection at risk of progression according to the AEMPS prioritisation criteria.
The objective of this article is to describe this established clinical pathway and to evaluate the first results of its implementation.
MethodsThis was a descriptive study of the implementation of a clinical pathway for early identification, referral and antiviral treatment of patients with SARS-CoV-2 infection and high risk of progression in our Department of Health (reference population: 280,000 inhabitants). The effectiveness of the clinical pathway for patients who received antiviral treatment at the COVID-19 day hospital (DH_COVID19) was evaluated.
Design and implementation of the clinical pathwayA clinical pathway was structured based on the identification and referral of patients, with the coordination of the Infectious Diseases Unit aimed at Primary Care, the Accident and Emergency Department and hospital specialties. The DH_COVID19 and a clinic for clinical follow-up were created in the facilities of the multi-specialty hospital of our centre, with care provided by two physicians from the Infectious Diseases Unit and three nursing staff (one for telephone follow-up), with partial dedication of approximately 20% of the working day.
Patient detection and referralAutomated detection. In order to facilitate the detection of patients with a shorter duration of infection, an automated detection system of candidates for treatment was designed through:
- -
Generation of a database of patients from the Department of Health (according to AEMPS prioritisation criteria): medical conditions or pharmacological treatment. This database (database I) was prepared from the Health Agency's outpatient care system and the Pharmacy Department registry (Table 1).
Table 1.Sources of records of immunosuppressed patients or those with other high-risk conditions used for preparing the reference database of the automated pathway.
Primary Care Registry (SIA) Diagnoses Solid organ transplant Bone marrow transplant Stage 5 chronic kidney disease (renal replacement therapy: haemodialysis or peritoneal dialysis) Human immunodeficiency virus infection Cystic fibrosis Down's syndrome >40 years Treatments: Primary Care prescription record Methotrexate Leflunomide 6-mercaptopurine Azathioprine Ciclosporin, mycophenolate, tacrolimus, sirolimus and everolimus Registry of Hospital Pharmacy Department and Outpatient Pharmacy Unit Myelotoxic chemotherapy (last 6 months) Drug administration in the last 6 months Anti-CD20 monoclonal antibodies Inhibitors of B cell proliferation T cell suppressor fusion proteins Interleukin 1 inhibitors Anti-CD52 monoclonal antibodies Sphingosine-1-phosphate receptor modulators Protein kinase inhibitors Janus kinase inhibitors - -
Daily generation by the Microbiology Department of patients diagnosed with SARS-CoV-2 infection (RT-PCR or antigen) (database II).
- -
Daily cross-referencing of both databases (I and II), which enabled the identification of SARS-CoV-2 infection in patients who were candidates for treatment. Review and evaluation by the Infectious Diseases Unit team.
Detection and referral from other specialties. Dissemination of information for referral to the DH_COVID19 of possible candidates for treatment in three areas:
- -
Hospital medical specialties with patients at risk of progression: the treatment and referral criteria were presented in a clinical session and by disseminating the information by email.
- -
Accident and Emergency Department: a checklist of criteria for treatment evaluation and referral to the DH_COVID19 was implemented, presented in clinical sessions.
- -
Primary Care: presentation of referral criteria in clinical sessions with the Primary Care PROA team (health centre coordinators, PROA advisers).
The referral was made by telephone or through interconsultation in the hospital computer system.
Continuous updating was carried out based on the new regulatory documents issued by the AEMPS and the Ministry of Health's General Directorate of Pharmacy and Medical Devices. An infographic with the treatment criteria and referral pathways available was created and published on the intranet and the PROA team's web page.
Assessment of patients at the COVID-19 Day HospitalInitial telephone assessment of the patient. Regardless of the referral pathway, the patient's medical history was reviewed, and after confirming the patient as a candidate for treatment, telephone contact was established for evaluating the symptoms during an appointment in the DH_COVID19.
Clinical assessment in the DH_COVID19. With direct clinical evaluation, blood tests, prescription and administration of treatment.
Treatment indication. The treatment indication was established based on the clinical conditions and the available/authorised drugs. These were: (1) sotrovimab, in accordance with the criteria established by the AEMPS; (2) remdesivir as an alternative for patients with other states of immunosuppression (not included in the AEMPS criteria), or the National Institutes of Health 1–2, and (3) nirmatrelvir/ritonavir according to the AEMPS v.3 update (24/03/2022).8
Treatment administration. The treatment was administered in the DH_COVID19. In exceptional cases, in dependent patients or those unable to travel, it was administered at home, through the Home Hospitalisation Unit.
Clinical follow-upIn the care of all patients, a treatment report was issued as part of the hospital's electronic medical record, accessible from Primary Care and from other centres of the Department of Health. Additionally, for patients referred by hospital medical specialties, telephone contact was made to inform them about the treatment administered.
A computer application was generated using automatic flowcharts provided by the AppSheet® platform (Google LLC's no-code app development platform) for data management with registration of the main variables and with automated appointment generation for telephone follow-up by nursing staff of the Infectious Diseases Unit.
Follow-up was conducted until the resolution of symptoms, with final contact on day 30 after the administration of treatment. In each telephone assessment, a follow-up report was issued in the electronic medical record.
Evaluation of resultsThe study included a retrospective cohort of patients referred and treated at the DH_COVID19 between 1 January and 30 June 2022. The implementation of the clinical pathway and its effectiveness were evaluated following the established protocol.
Explanatory variablesDemographic variables (age, gender, ethnicity/race), referral system, associated comorbidity, treatment indication criteria (stratified according to the AEMPS prioritisation criteria), vaccination status (number of doses administered), anti-protein S antibody level quantified in BAU/mL (SARS-CoV-2 IgII Quant Reagent Kit, Abbott) and previous SARS-CoV-2 infection were all considered.
Outcome variablesTo evaluate the clinical pathway, the number of clinical sessions was recorded. The total number of patients referred, the means of referral and the percentage in whom treatment was indicated were evaluated.
To evaluate the effectiveness of the treatment, hospitalisation and/or death at 30 days (excluding those occurring in the first 24 h of treatment) were considered as the main variable, and as secondary variables, the presence of grade 2−3 toxicity and discontinuation of treatment.
Statistical analysisCategorical variables are expressed as frequencies and their percentages, while continuous variables are expressed through means and standard deviations or medians and interquartile range (IQR), depending on whether or not they follow a normal distribution. The index date was the DH_COVID19 assessment date, and the final follow-up date was at 30 days, unless censored. The statistical analysis of the results was performed with the program IBM® SPSS® version 22 (IBM Corp., Armonk, NY). A p-value <0.050 was defined as statistically significant.
Ethical aspectsThe study protocol was approved by the Ethics Committee of the hospital (PI2021) and, as it was a retrospective study, exemption from informed consent was requested. The Guideline for Good Clinical Practice and the Declaration of Helsinki were followed.
ResultsImplementation and evaluation of the clinical pathwayIn the first month of the study, a clinical pathway for the referral of patients who were candidates for treatment was developed with the preparation of infographics for the Accident and Emergency Department (Appendix B, Fig. S1) and Primary Care (Appendix B, Fig. S2), with dissemination in the Department of Health (by email, corporate intranet and dissemination on the website www.proabalmis.com). Six clinical sessions were held with the Accident and Emergency Department, five with hospital medical specialties and three with Primary Care PROA coordinators and advisers.
There is no complete record of patients referred according to the clinical pathway during the entire study period. Systematic registration for the automated detection pathway began on 1 February 2022, with treatment of 50.3% of the detected patients (73/145), and from 1 May for the Accident and Emergency Department and Primary Care pathways, with treatment of 60.5% (72/119) and 42.3% (33/78) of the referred patients, respectively. Referral from the different specialties was by telephone and there is no record until June 2022.
Administration and effectiveness of treatmentFrom 1 January to 30 June 2022, treatment was indicated in 262 patients: 53.4% women, with a median age of 60 years (IQR 46−71.3). Of these, 84.4% had the complete vaccination schedule (with booster dose). Meanwhile, 68.3% exhibited states of immunosuppression, with associated comorbidities in more than half of the patients (Table 2).
Characteristics of patients treated at the COVID-19 day hospital (N = 262).
| n (%) | |
|---|---|
| Demographic aspects | |
| Age (years), median (IQR) | 60 (46−71.3) |
| Gender, female | 140 (53.4) |
| Nationality, not Spanish | 18 (6.9) |
| Previous COVID | 17 (6.5) |
| Vaccination (with a booster dose) | 221 (84.4) |
| Referral pathway | |
| Automated screening | 86 (32.8) |
| Hospital A&E | 72 (27.5) |
| Hospital specialties | 71 (27.1) |
| Primary Care | 33 (12.6) |
| History, comorbidity | |
| Arterial hypertension | 125 (47.7) |
| Diabetes mellitus | 42 (16.0) |
| Body mass index >30 | 49 (18.7) |
| Smoking | 81 (31.0) |
| Cardiovascular | 41 (15.6) |
| Chronic kidney diseasea | 32 (12.2) |
| Dialysis | 22 (8.4) |
| Cardiovascular disease | 13 (5.0) |
| Chronic lung disease | 9 (3.4) |
| Asthma | 10 (3.8) |
| Immunosuppression | 179 (68.3) |
| Treatment criteria | |
| Immunosuppressed | |
| Biologic immunomodulators | 116 (43.8) |
| Anti-CD20 | 46 (17.6) |
| Anti-TNF | 43 (16.4) |
| Other biologics | 11 (4.2) |
| JAK inhibitors | 8 (3.1) |
| Protein kinase inhibitors | 8 (3.0) |
| Solid organ transplantb | 25 (9.5) |
| Neoplasm with active CT | 17 (6.5) |
| Non-biologic immunomodulators | 12 (4.6) |
| HIV infection <200 cells CD4/μl | 3 (1.1) |
| Primary immunodeficiency | 3 (1.1) |
| Haematopoietic stem cell transplantation | 1 (0.4) |
| Other causes of immunosuppression | 5 (1.9) |
| Other treatment criteria | |
| Advanced age, vaccination status and comorbidities | 59 (22.5) |
| Haemodialysis | 14 (5.3) |
| Not vaccinated | 8 (3.1) |
| Cystic fibrosis | 1 (0.4) |
| Down's syndrome >40 years | 1 (0.4) |
| Treatment | |
| Days after symptom onset, median (IQR) | 4 (3−5) |
| Remdesivir 3 days | 124 (47.3) |
| Nirmatrelvir/ritonavir | 94 (35.9) |
| Sotrovimab | 35 (13.4) |
| Combined treatments | 9 (3.4) |
| Toxicity | 49 (18.7) |
| Gastrointestinal | 44 (16.8) |
| Other | 5 (1.9) |
| Discontinuation of treatment due to toxicity | 7 (2.7) |
| Prioritisation levels according to NIH | |
| 1. Immunosuppressed and unvaccinated >75 years or >65 years with risk factors | 179 (68.3) |
| 2. Unvaccinated >65 years or <65 years with risk factors | 8 (3.1) |
| 3. Vaccinated >75 years or >65 years with risk factors | 47 (17.9) |
| 4. Vaccinated >65 years or <65 years with risk factors | 28 (10.7) |
| Clinical course | |
| Hospitalisation | 16 (6.1) |
| Hospitalisation for COVID-19 | 10 (3.8) |
| Death at 30 days | 0 (0) |
CT, chemotherapy; HIV, human immunodeficiency virus; IQR, interquartile range; JAK, Janus kinase family; NIH, National Institutes of Health; TNF, tumour necrosis factor.
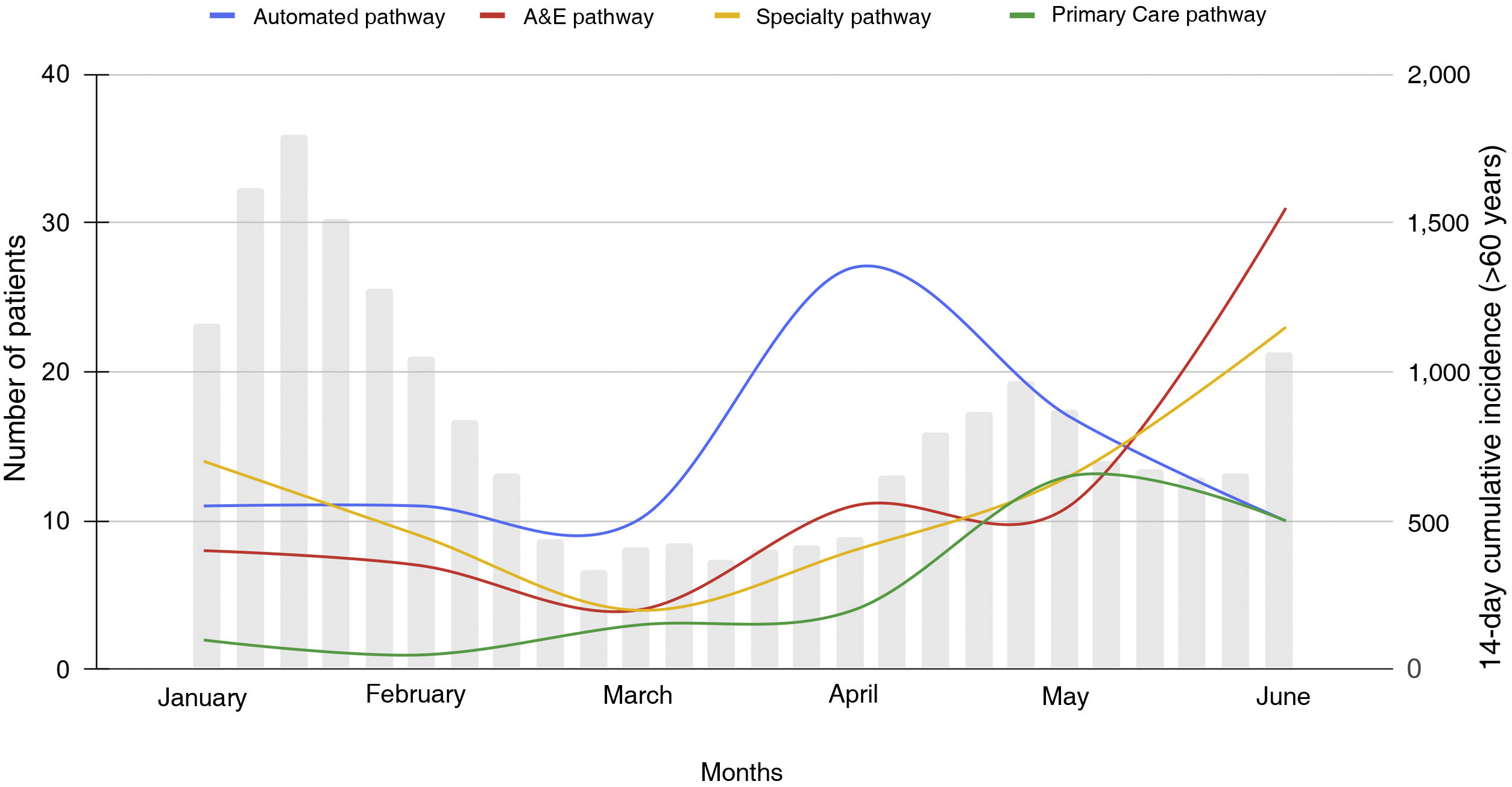
These patients were referred with similar percentages from the automated detection system, hospital medical specialties and the Accident and Emergency Department (32.8%, 27.1% and 27.5%, respectively) and from Primary Care (12.6%) over the study period, but with differing distribution throughout the six months (Fig. 1). Automated detection was the primary referral pathway until 1 May 2022.
All referred patients from all referral pathways could be contacted; in five cases (1.87%) the patient refused the treatment.
The criterion for indicating treatment, according to the high-risk conditions established in the AEMPS criteria, was mainly immunosuppression (68.3%). In the rest, it was a combination of age, vaccination status and comorbidity (Table 2).
Treatment was administered at a median of four days (IQR 3−5) after the onset of symptoms. In total, 47.3% of patients received remdesivir (n = 124), 35.9% nirmatrelvir/ritonavir (n = 94), 13.4% sotrovimab (n = 25) and 2.4% combination therapy (n = 9). The characteristics of the patients according to the drug administered are detailed in Table 3.
Characteristics of patients treated at the COVID-19 day hospital according to the drug administered.
| Remdesivir | Nirmatrelvir/ritonavir | Sotrovimab | |
|---|---|---|---|
| N | 124 | 94 | 35 |
| Age (years), median (IQR) | 62 (50.3−71) | 62 (44.8−74.5) | 53 (42−65) |
| Gender, female | 56 (45.2) | 59 (62.8) | 18 (51.4) |
| Vaccination with booster dose | 104 (83.9) | 80 (85.1) | 30 (85.7) |
| Post-vaccination Ab >260 BAU (n = 140) | 5/12 (42.7) | 39/93 (41.5) | 6/35 (17.1) |
| Previous SARS-CoV-2 infection | 6 (4.8) | 7 (7.4) | 1 (2.9) |
| Comorbidity | 80 (64.5) | 36 (38.3) | 15 (42.9) |
| Treatment indication criteria | |||
| Immunosuppression | 83 (66.9) | 56 (59.6) | 32 (91.4) |
| Comorbidity/vaccination | 41 (33.1) | 38 (40.4) | 3 (8.6) |
| Toxicity grade 2−3 | 2 (1.6) | 42 (44.7) | 1 (2.9) |
| Hospitalisation | |||
| Day 30 post-treatment | 8 (6.5) | 3 (3.2) | 3 (8.6) |
| Due to COVID progression | 5 (4) | 2 (2.1) | 1 (2.9) |
| 30-day mortality | 0 | 0 | 0 |
Number of patients (%), unless otherwise specified. Nine patients on combination therapy were excluded.
Ab, antibodies; IQR, interquartile range.
Sixteen patients (6.1%) required hospital admission, 10 due to progression of SARS-CoV-2 infection (3.8% of the total), with a median hospital stay of nine days (IQR 6.8–13.3). No patients required non-invasive ventilation or admission to the critical care unit, and none of them died.
Forty-nine patients (18.7%) exhibited toxicity, which was grade 2 in 42 patients (16.03%) and grade 3 in six patients (2.29%), mainly in the group treated with nirmatrelvir/ritonavir, with dysgeusia and metallic taste in 44 patients (89.8%) and suspension of treatment in six of them (6.1%), due to grade 3 toxicity. Three patients had grade 2 gastrointestinal intolerance due to remdesivir, with suspension of treatment after the first dose in one patient, and another patient treated with remdesivir showed a slight elevation of transaminases (AST and ALT × 2), but completed the treatment cycle. One patient experienced nausea with sotrovimab.
There were no losses to follow-up in the nursing team's telephone assessments until resolution of the symptoms and all the patients or their relatives could be contacted on day 30 post-treatment to evaluate the outcome.
DiscussionIn our experience, the implementation of a structured clinical pathway for outpatient antiviral treatment has enabled the identification, referral and effective treatment of patients with SARS-CoV-2 infection and a high risk of progression to severe disease.
During the first months of 2022, the treatment of acute SARS-CoV-2 infection was a challenge in our healthcare system. First, because its indication was determined by criteria established in the successive AEMPS' updates. Second, due to the absence of an oral alternative (available as of 24 March 2022), which made it difficult to administer. Third, due to the need to disseminate information among different specialists who care for patients. Fourth, because the appropriate indication should be guaranteed based on the available evidence, as well as subsequent follow-up. And lastly, due to the narrow time frame after the onset of symptoms that guarantees treatment efficacy, requiring very agile care pathways.
These difficulties led us to design and implement this clinical pathway. This has the potential to improve health outcomes, since it makes it possible to reduce variability, improve the quality of care and optimise the treatment response for specific groups, as well as promoting the incorporation of available evidence into clinical practice quickly and efficiently. But its impact depends on an appropriate design, good coordination and a correct approach towards overcoming the difficulties its implementation poses.9
The four principles that are proposed and that have served as a guide for the development of the clinical pathway, according to Jabbour et al.,10 are: (1) a structured and multidisciplinary intervention plan should be prepared; (2) scientific evidence or general clinical guidelines should be passed on to local healthcare facilities; (3) steps in the treatment course should be detailed in the form of a plan and algorithm, and (4) standardisation of care should be sought for specific populations.
During these initial six months we evaluated around 500 patients, of whom 262 were treated for very high-risk conditions. Special mention should be made of the automated detection system, which identified more than a third of the treated patients very early (<48 h after diagnosis). Coordination with the different care units (Accident and Emergency Department, hospital specialties and Primary Care) and the Microbiology and Pharmacy departments ensured its proper implementation and development.
There is little experience in the scientific literature regarding clinical pathways for non-hospitalised patients with SARS-CoV-2 infection. Only Manciulli et al.11 describe a clinical pathway of referral for detection of Primary Care patients to a specific treatment unit, with a purely economic analysis. They raise the need to dedicate COVID-19 specialists to outpatient treatment and the development of a care structure with "local health teams" or Primary Care teams.
Regarding the effectiveness of the treatment, although we do not have a comparator group that allows for its proper evaluation, the absence of mortality, a 6% overall hospital admission rate and 3.8% due to progression of COVID-19, seem to point towards the effectiveness of the treatments, particularly given the very high-risk population.
In populations infected by Omicron and predominantly vaccinated, such as ours, the evidence of these treatments in "real life" is scarce and comes from observational studies that are non-comparable, given the different characteristics of the populations included.
Experience with sotrovimab is mainly focused on the population with severe immunosuppression. Martin-Blondel et al.12 describe 116 patients treated with sotrovimab (mean age 55 years, 77% severe immunosuppression, and 77% vaccinated with booster dose); they reported a hospitalisation incidence of 3%, with no deaths. In a series of 300 solid organ transplant patients, 109 of them treated with sotrovimab (mean age 55 years, 65% with three or more doses of vaccine), Solera et al.13 demonstrated a reduced risk of hospitalisation (RR 0.24, 0.1−0.59). In another series of 125 transplant recipients, Chavarot et al.14 compared 25 who received sotrovimab with the rest who were not treated, with a hospital admission rate of 16% and 35%, and a mortality rate of 0% and 4%, respectively.
With regard to nirmatrelvir/ritonavir, large population studies have recently been published. In the first, carried out in 5,282 low-risk patients (58% under 65 years of age, 72% vaccinated and 43% without comorbidity), less than 1% were hospitalised and/or attended the Accident and Emergency Department.15 In another study of 4,737 patients treated with nirmatrelvir/ritonavir compared with 175,214 untreated patients (75% vaccinated, population with at least one risk factor), multivariate analysis demonstrated an 80% reduction in the risk of hospitalisation for vaccination and 40% for treatment, regardless of vaccination status. The greatest impact was observed in older patients, with neoplasia or associated immunosuppression.16 One last series conducted in Israel compared 3,902 treated patients with 109,254 untreated patients, all with some risk factor for progression (90% with prior immunisation), demonstrating a reduction in hospitalisations (adjusted HR 0.27, 95% CI 0.15−0.49) and deaths (adjusted HR 0.21, 95% CI 0.05−0.82) in people over 65 years of age.17
To our knowledge, there is no published experience with remdesivir, beyond the PINETREE clinical trial.4
These studies have a very different population from ours, which adhered to the treatment conditions established by the AEMPS. In our experience, as well as in the series discussed, the safety of all the treatments administered is striking, with a minimal incidence of discontinuation.
Our study has limitations inherent to its single-centre, retrospective observational design, and to the limited sample size. However, the existence of a standardised clinical history and the absence of losses to follow-up guarantees the quality of the information. The study underestimates the percentage of patients treated versus those referred, since a systematic registry of patients referred from the different levels of care and treated on an outpatient basis was not carried out from the beginning. This was because the outpatient treatment strategy began in January 2022 during the epidemic wave and we initially had difficulties with patient registration and the referral pathways were not fully structured, being managed mainly through telephone contact. Finally, the lack of a control group, as we are not currently aware of the outcomes of the untreated patients, does not allow for a proper evaluation of the effectiveness of the treatment.
As for the future, we believe there is a need to develop and optimise our current pathway in two areas: the decentralisation of prescription treatments from the different levels of care, which is currently ongoing; and the need for information for the vulnerable population, with a high frequency of self-diagnosis and delay in seeking healthcare.
ConclusionThe creation and implementation of a clinical pathway for patients with SARS-CoV-2 infection is effective and facilitates early accessibility and equity of currently available treatments for vulnerable patients who are eligible for treatment based on the recommendations.
The leadership of expert clinicians (infectious disease specialists) within multidisciplinary teams in infectious diseases (PROA) ensures its optimised implementation in our healthcare system.
NoteHPC and EM have full access to the data, which may be provided upon proper and justified request.
FundingThis research did not receive any specific grants from funding agencies in the public, commercial or nonprofit sectors.
AuthorshipOriginal manuscript: HPC, SOR, JMRR, EM. Writing, review and editing: HPC, SOR, JMRR, EM, PGA, JCRD, OMP, EC, PCS, GR, PL, MA, IM, VB. Conceptualisation: HPC, EM, JMRR. Research: HPC, SOR, EM, PGA. Methodology: EM, JMR. Formal analysis: HPC, SOR, JMRR, EM. Project management: EM.
Conflicts of interestEM has participated in presentations funded by GILEAD, GSK and Pfizer. The rest of the authors declare that they have no conflict of interest.
We would like to thank all the nursing staff of the DH_COVID19, Lourdes Rodríguez, and of the Infectious Diseases Unit, María Jesús Alcazo and María José de la Encarnación, for their important collaboration in the implementation of this clinical pathway.