Pneumocystis jirovecii pneumonia (PJP) is a life-threatening condition in immunocompromised children. Our aim is to analyze the epidemiologic and clinical characteristics of PJP cases in our setting, describing the prognosis and related risk factors.
MethodsRetrospective study including all pediatric patients (≤18 years) with PJP admitted to our hospital (January 1989–December 2016). Case definition: patient with acute pneumonitis and P.jirovecii detection in bronchoalveolar lavage or tracheal aspirate using methenamine silver or direct antibody fluorescence staining, or Real-Time Polymerase Chain Reaction.
ResultsTwenty-five cases (0.9 cases/year) were identified. Median age was 2.2 years (interquartile range: 0.5–12.3), 64% were male, and 12% were receiving appropriate antimicrobial prophylaxis. Cytomegalovirus coinfection was detected in 26% cases. The most common underlying diseases were primary immunodeficiencies (36%) and 16% were human immunodeficiency virus (HIV)-infected children. Eighteen were admitted to the pediatric intensive care unit (PICU) and overall 30-day mortality was 20% (31.25% in HIV non-infected vs 0% in HIV-infected patients; OR: 0.33, 95% CI: 0.02–7.24, p=0.55). Clinical outcome was worse in girls and those patients requiring adjuvant steroid therapy. HIV non-infected patients, higher initial LDH, younger age and shorter time elapsed between diagnosis of PJP and the underlying disease were identified as risk factors to be admitted to the PICU (p=0.05, p=0.026, p=0.04 and p=0.001 respectively).
ConclusionAccompanying the widespread use of combined antiretroviral therapy, PJP has been diagnosed almost exclusively in HIV non-infected children at our institution. Moreover, significant higher morbidity rates associated with PJP are seen in this group of patients.
La neumonía por Pneumocystis jirovecii (PJP) es una enfermedad potencialmente letal en niños inmunocomprometidos. Nuestro objetivo es analizar las características epidemiológicas y clínicas de la PJP, describiendo el pronóstico y los factores de riesgo.
MétodosEstudio retrospectivo (enero 1989-diciembre 2016) de pacientes pediátricos (≤18 años) con PJP. Definición de caso: paciente con neumonitis aguda y detección de P. jirovecii en lavado broncolaveolar o aspirado traqueal usando tinción con plata-metenamina o inmunofluorescencia directa, o reacción en cadena de polimerasa en tiempo real.
ResultadosSe identificaron veinticinco casos (0,9 casos/año); edad mediana: 2,2 años (rango intercuartílico: 0,5-12,3), 64% de sexo masculino, y 12% bajo profilaxis anti-PJP. La coinfección por citomegalovirus se demostró en el 26%. Las enfermedades subyacentes más frecuentes fueron las inmunodeficiencias primarias (36%) y el 16% estaban infectados por el VIH. Dieciocho ingresaron en Cuidados Intensivos Pediátricos (UCIP) y la mortalidad global a los 30 días fue del 20% (31,25% en VIH- vs 0% VIH+; OR: 0,33 95%CI 0,02-7,24 p=0,55). El pronóstico fue peor en niñas y en aquellos que recibieron tratamiento adyuvante con corticoides. Se identificaron como factores de riesgo para ingreso en UCIP la ausencia de infección por VIH, valores iniciales elevados de LDH, menor edad y un período más corto entre el diagnóstico de PJP y la enfermedad subyacente (p=0,05, p=0,026, p=0,04 y p=0,001, respectivamente).
ConclusionesTras la aplicación generalizada de la terapia antirretroviral, la PJP se diagnostica casi exclusivamente en niños no infectados por el VIH en los que, además, se identificó una mayor morbilidad.
Pneumocystis jirovecii (PJ) is an opportunistic yeast-like fungus with worldwide distribution that can cause overt pneumonia in immunocompromised hosts and results in death without treatment. Disseminated infection has also been described. In the 1940s it was described as a cause of pneumonia in malnourished and institutionalized children in Europe,1 and in the 1970s it was often diagnosed in children with hematologic malignancies.2 It is well recognized that children born with certain primary immunodeficiencies (PID), such as severe combined immunodeficiency (SCID),3 idiopathic T CD4+ lymphopenia,4 X-linked hyper-IgM syndrome,5 nuclear factor-kappa B essential modulator (NEMO) deficiency,6 and Wiskott-Aldrich syndrome7 among others,8–13 are also susceptible to develop P. jirovecii pneumonia (PJP).
During the human immunodeficiency virus (HIV) outbreak in the 1980s, the incidence of PJP increased dramatically14 in children as well as adults, becoming the most common acquired immunodeficiency syndrome (AIDS)-defining condition during the first year of life in HIV-infected infants.15 Systematic use of antimicrobial prophylaxis with trimethoprim-sulfamethoxazole in the era of highly active – or combined - antiretroviral therapy (HAART or c-ART more recently) has led to a considerable decrease in the incidence of PJP in this population.16
However, the concomitant growing use of immunosuppressive agents and novel biologic therapies has led to a change in the classic scenario of patients affected by PJP, with an increasing incidence in other types of immunocompromised hosts. This has resulted in the publication of specific prophylactic guidelines for selected groups of patients, which have provided an estimated risk reduction of 85%.17–19
The aim of this study was to analyze the epidemiologic and clinical characteristics of PJP in a single center during the last three decades, describing the outcomes and related risk factors in an exclusively pediatric cohort, spanning in time from AIDS to post- HAART era.
MethodsStudy settingVall d’Hebron Children Hospital, Barcelona (Catalonia, Spain), is a 284-bed tertiary referral center with around 3000 admissions per year and 41 critical care beds (including neonates). Our center provides medical care to a large population of immunosuppressed pediatric patients, including those with primary immunodeficiencies, HIV infection, cancer patients (including those requiring stem cell transplantation), and solid organ transplant recipients (kidney, liver, heart, and lung transplantation).
Study design and definitionsWe retrospectively collected the epidemiological, microbiological, clinical, and biological data of all pediatric PJP cases (≤18 years of age) occurring in the period from January 1989 to December 2016 (28 years). Patients were identified using the International Classification of Diseases 9 code, 136.3.
Case definition: any pediatric patient with acute respiratory failure and PJ detection in bronchoalveolar lavage (BAL), tracheal aspirate, and/or lung biopsy by positive cytology (Gomori methenamine-silver stain), positive direct antibody fluorescence staining (Meryfluor Pneumocystis, Meridian Bioscience Inc., Ohio, USA; sensitivity 96% and specificity 95%) or positive semi-quantitative real-time Polymerase Chain Reaction (RT-PCR) testing using dihydropteroate synthase as the target.20 Our previously validated, in-house RT-PCR technique is considered positive if the cycle threshold (Ct) value is <30. Ct values>32 are considered as colonization and excluded. Ct values between 30 and 32 are considered a “grey zone” and require validation with the patients’ clinical status (3 patients in the cohort). The RT-PCR showed a sensitivity of 83.3%, specificity of 93.1%, positive predictive value of 85.7%, and negative predictive value of 92.9% in previous in-house studies (Martín-Gómez, MT; unpublished data).
Data collectionDemographic data, clinical features, microbiological and radiological findings, therapeutic approach and clinical outcome were recorded. Length of hospital stay, need for admission to the pediatric intensive care unit (PICU), length of PICU stay, need for mechanical ventilation, duration of ventilatory support, and 30-day, 60-day, and 90-day mortality rates were also documented. Death was considered as PJP-related if clinical records included PJP as a cause of death and respiratory weaning was not achieved after diagnosis.
Statistical analysisQualitative variables are expressed as the frequency and percentage. Quantitative variables are expressed as the median and interquartile range, as none were normally distributed. Univariate analysis was performed (chi-square or Fisher exact test for qualitative variables, Mann–Whitney for quantitative variables, and Spearman for correlation tests). Since multivariate analysis was not feasible due to the relatively low number of cases included, stratified subanalyses by gender, use of adjuvant steroids, and HIV infection status were carried out. In all statistical comparisons, significance was set at a p-value<0.05. SPSS v22 software (IBM, Armonk, New York, USA) was used for the data analysis.
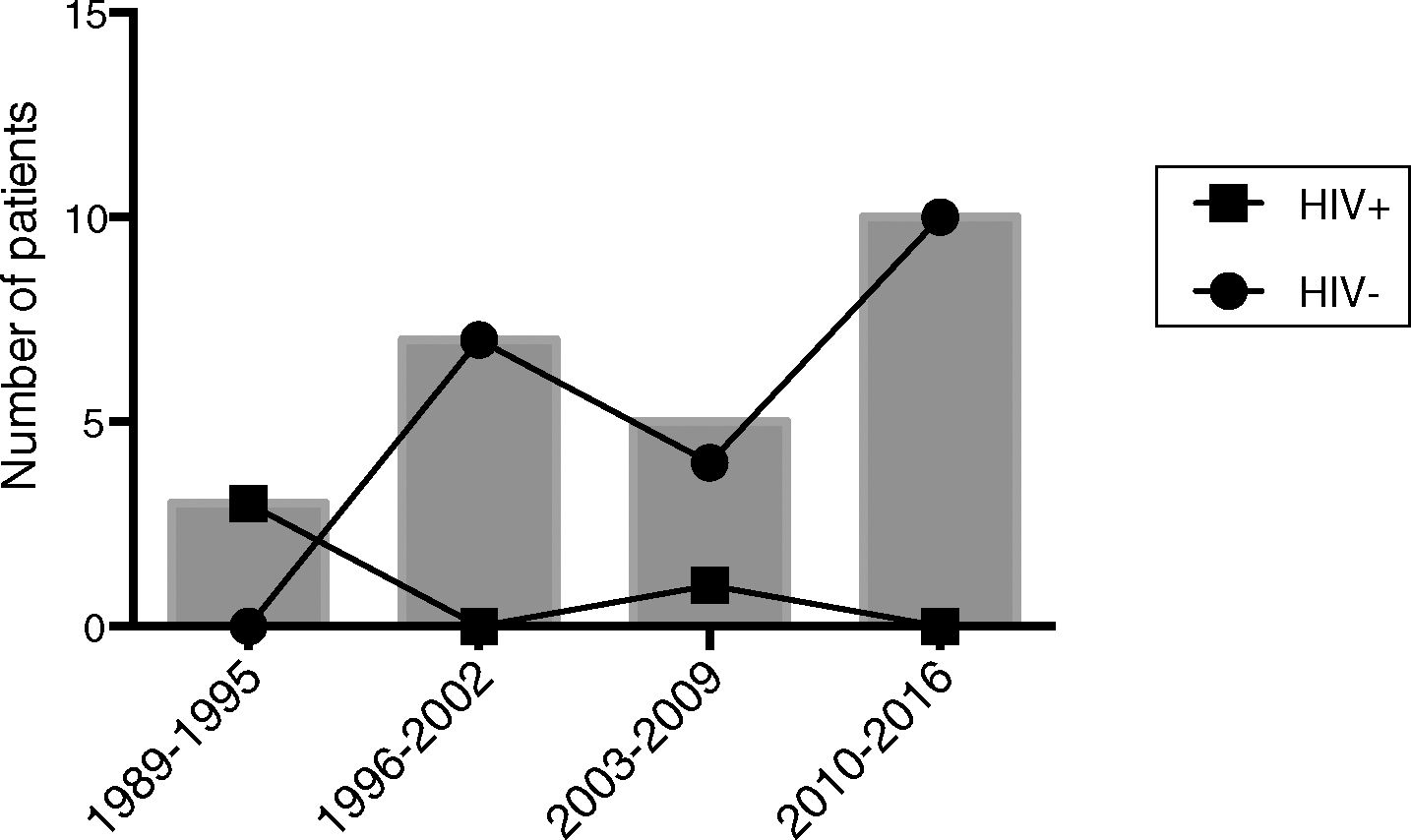
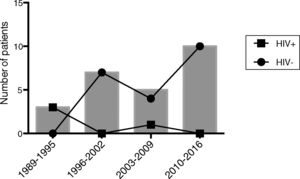
ResultsTwenty-seven cases were initially identified along the study period. Two cases were excluded (a boy with X-linked agammaglobulinemia and an HIV-infected girl born in Thailand) because of clinical data incompleteness (Table 1). Median age at the diagnosis was 2.2 years (interquartile range [IQR] 0.5–12.3). Sixteen patients (64%) were males. Overall incidence was 0.01 cases per 1000 admissions/year, and 10 cases (40%) were diagnosed in the last quarter of the period (2010–2016). This yielded a surge in the incidence of 0.07 cases per 1000 admissions/year, doubling the incidence of the previous period (Fig. 1). No disseminated or extrapulmonary forms of PJ infection were diagnosed.
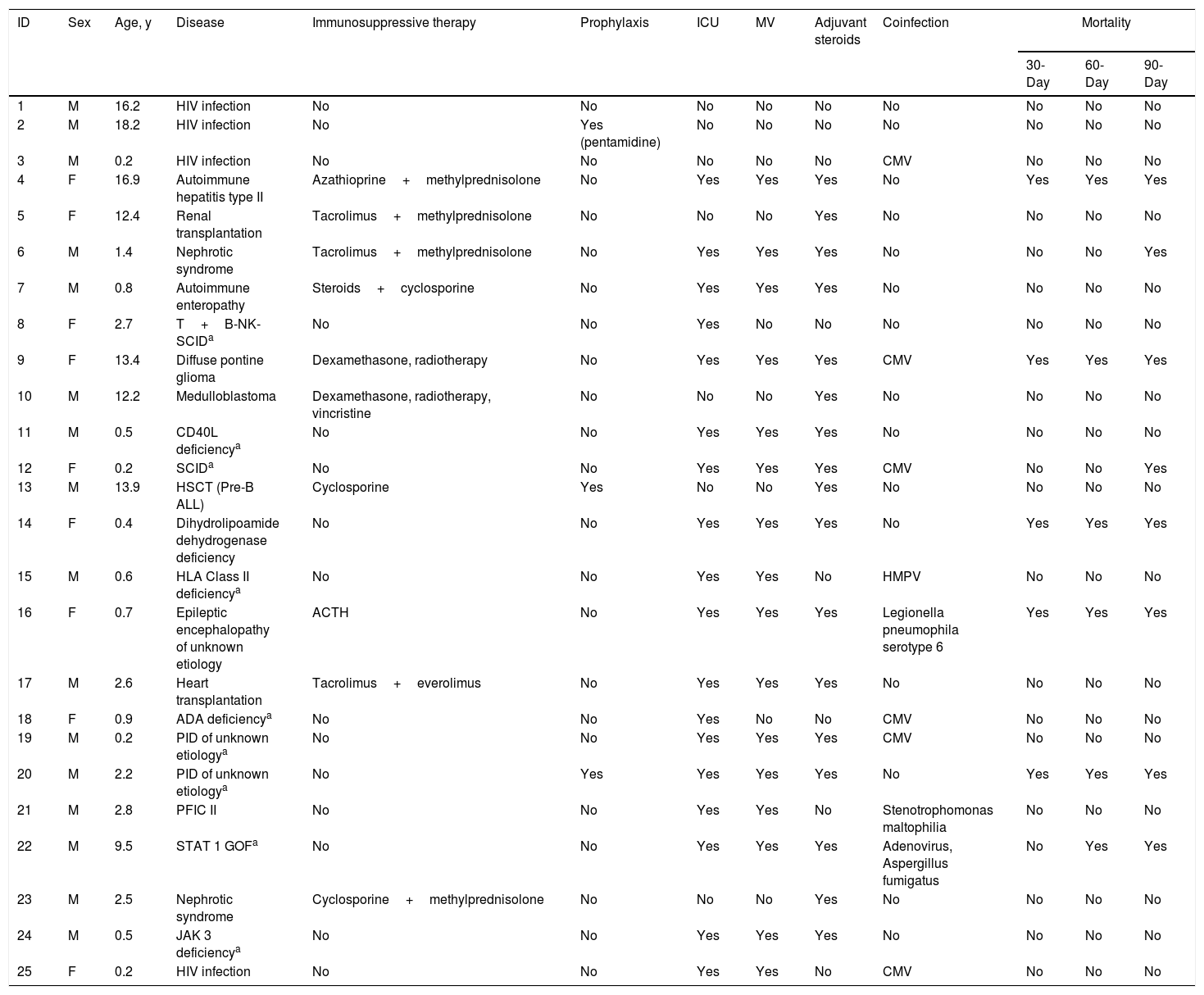
Clinical and demographic features of the study patients.
| ID | Sex | Age, y | Disease | Immunosuppressive therapy | Prophylaxis | ICU | MV | Adjuvant steroids | Coinfection | Mortality | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30-Day | 60-Day | 90-Day | ||||||||||
| 1 | M | 16.2 | HIV infection | No | No | No | No | No | No | No | No | No |
| 2 | M | 18.2 | HIV infection | No | Yes (pentamidine) | No | No | No | No | No | No | No |
| 3 | M | 0.2 | HIV infection | No | No | No | No | No | CMV | No | No | No |
| 4 | F | 16.9 | Autoimmune hepatitis type II | Azathioprine+methylprednisolone | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 5 | F | 12.4 | Renal transplantation | Tacrolimus+methylprednisolone | No | No | No | Yes | No | No | No | No |
| 6 | M | 1.4 | Nephrotic syndrome | Tacrolimus+methylprednisolone | No | Yes | Yes | Yes | No | No | No | Yes |
| 7 | M | 0.8 | Autoimmune enteropathy | Steroids+cyclosporine | No | Yes | Yes | Yes | No | No | No | No |
| 8 | F | 2.7 | T+B-NK-SCIDa | No | No | Yes | No | No | No | No | No | No |
| 9 | F | 13.4 | Diffuse pontine glioma | Dexamethasone, radiotherapy | No | Yes | Yes | Yes | CMV | Yes | Yes | Yes |
| 10 | M | 12.2 | Medulloblastoma | Dexamethasone, radiotherapy, vincristine | No | No | No | Yes | No | No | No | No |
| 11 | M | 0.5 | CD40L deficiencya | No | No | Yes | Yes | Yes | No | No | No | No |
| 12 | F | 0.2 | SCIDa | No | No | Yes | Yes | Yes | CMV | No | No | Yes |
| 13 | M | 13.9 | HSCT (Pre-B ALL) | Cyclosporine | Yes | No | No | Yes | No | No | No | No |
| 14 | F | 0.4 | Dihydrolipoamide dehydrogenase deficiency | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 15 | M | 0.6 | HLA Class II deficiencya | No | No | Yes | Yes | No | HMPV | No | No | No |
| 16 | F | 0.7 | Epileptic encephalopathy of unknown etiology | ACTH | No | Yes | Yes | Yes | Legionella pneumophila serotype 6 | Yes | Yes | Yes |
| 17 | M | 2.6 | Heart transplantation | Tacrolimus+everolimus | No | Yes | Yes | Yes | No | No | No | No |
| 18 | F | 0.9 | ADA deficiencya | No | No | Yes | No | No | CMV | No | No | No |
| 19 | M | 0.2 | PID of unknown etiologya | No | No | Yes | Yes | Yes | CMV | No | No | No |
| 20 | M | 2.2 | PID of unknown etiologya | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| 21 | M | 2.8 | PFIC II | No | No | Yes | Yes | No | Stenotrophomonas maltophilia | No | No | No |
| 22 | M | 9.5 | STAT 1 GOFa | No | No | Yes | Yes | Yes | Adenovirus, Aspergillus fumigatus | No | Yes | Yes |
| 23 | M | 2.5 | Nephrotic syndrome | Cyclosporine+methylprednisolone | No | No | No | Yes | No | No | No | No |
| 24 | M | 0.5 | JAK 3 deficiencya | No | No | Yes | Yes | Yes | No | No | No | No |
| 25 | F | 0.2 | HIV infection | No | No | Yes | Yes | No | CMV | No | No | No |
Primary immunodeficiency.
Abbreviations: ACTH, adrenocorticotropic hormone; ADA, adenosine deaminase; AIDS, acquired immunodeficiency syndrome; ALL, acute lymphoblastic leukemia; CMV, cytomegalovirus; HMPV, human metapneumovirus; ICU, intensive care unit; JAK 3, Janus kinase 3; MV, mechanical ventilation; PFIC, type II, progressive familial intrahepatic cholestasis type II; SCID, severe combined immunodefiency; STAT 1 GOF, signal transducer and activator of transcription 1 gain-of-function.
All affected children had an underlying disease, the most common being PID in 9 patients. Four patients were HIV-infected, 2 had a solid tumor, 2 had undergone solid organ transplantation (heart and kidney, respectively) and the remaining three were 1 hematopoietic stem cell transplantation, 1 familial intrahepatic cholestasis type II, and 1 dihydrolipoamide dehydrogenase deficiency, respectively. Five additional patients were on immunosuppressive therapy for a non-malignant disease.
Globally, ten patients (40%) were receiving an active immunosuppressive regimen at the time of the diagnosis: 6 were receiving steroids plus other immunosuppressant drugs (cyclosporine, mammalian target of rapamycin (mTOR) inhibitors or cytostatic agents), 1 had dual mTOR inhibitor-based therapy, and 3 had a single drug regimen (steroids alone plus radiotherapy, adrenocorticotropic hormone (ACTH), and cyclosporine, respectively). None of the patients with autoimmune diseases was receiving a treatment with biologicals.
Of note, 3 patients (12%) developed PJP while receiving an adequate prophylactic regimen: oral trimethoprim-sulfamethoxazole in 2 and nebulized pentamidine in 1. Among HIV-infected patients, both infants that developed PJP were diagnosed of HIV infection 8 days after and 11 days before their HIV diagnosis. Both infected teenagers developed PJP due their poor immunologic situation (<200CD4/mm3). However, assessment of proper adherence was not possible due to the retrospective nature of the study, and drug susceptibility studies were not performed.
With regard to PJP treatment, 22 patients (88%) were given trimethoprim-sulfamethoxazole alone, 2 received a sequential treatment (trimethoprim-sulfamethoxazole, followed by pentamidine due to clinical failure), and 1 received trimethoprim plus dapsone due to severe neutropenia. Median duration of treatment was 21 days (IQR 21–27.5). Steroids were given as adjuvant therapy in 17 patients (68%). There were no cases of immune reconstitution syndrome in our cohort.
Median length of hospital stay was 44 days (IQR 21–96). Eighteen patients (72%) were admitted to the PICU, and the median length of stay was 19 days (IQR 12.5–34.5). Sixteen PICU patients underwent mechanical ventilation, with a median duration of 14 days (IQR 6.5–34.3).
Thirty-day, 60-day, and 90-day mortality rates were 20%, 24%, and 32%, respectively. To note, there were no deaths in HIV-infected patients.
Twenty-four (96%) cases were identified from bronchoalveolar lavage material and 1 from tracheal aspirate. Direct antigen immunofluorescence staining techniques were positive in 17 patients (68%), and cytology in 6 (24%). The 2 remaining patients were diagnosed by RT-PCR. Only one lung biopsy was performed. Cytomegalovirus (CMV) was the most frequently identified co-infecting agent (26% of cases). Coinfections were not associated with higher mortality (p=1, p=0.65, and p=0.67 for 30-day, 60-day, and 90-day mortality, respectively), PICU admission (p=0.18), or mechanical ventilation requirement (p=0.23).
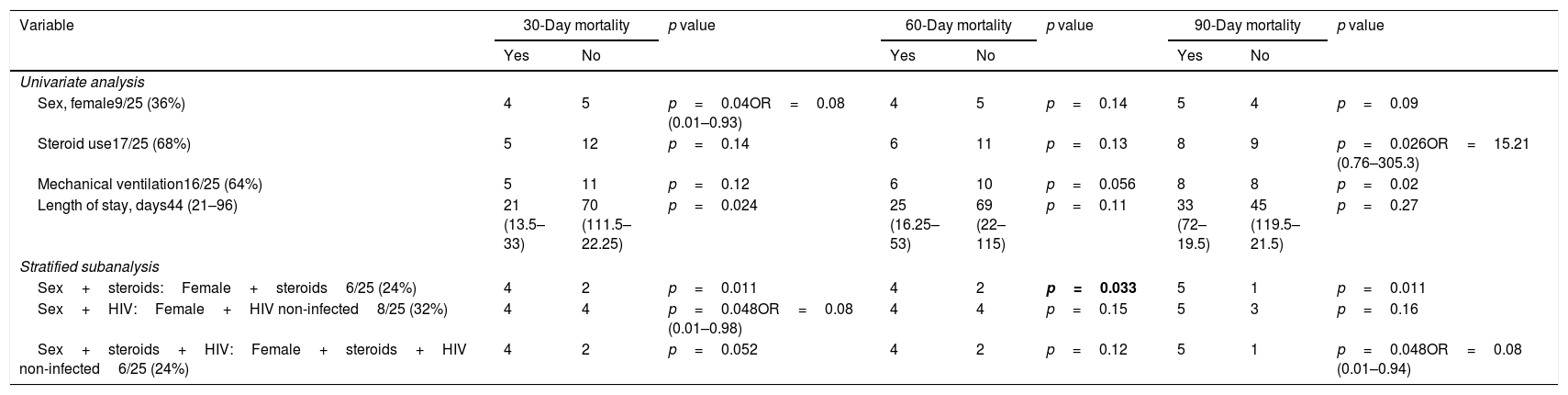
On univariate analysis of the patient characteristics and treatment modalities, female sex and shorter overall length of stay were associated with higher 30-day mortality. The need of mechanical ventilation and the use of steroids were significantly associated with higher 90-day mortality. Stratified subanalysis showed that HIV non-infected children or use of steroids in girls were associated with higher 30-day, 60-day and 90-day (in the case of steroids) mortality (Table 2). Although only HIV non-infected patients died, difference was not statistically significant again probably due to the relative low number of cases (30-day mortality: 31.25% in HIV non-infected vs 0% in HIV-infected patients; OR: 0.33 95%CI 0.02–7.24 p=0.55).
Prevalence of significant (and marginally significant) risk factors and variables associated with 30-day, 60-day, and 90-day mortality.
| Variable | 30-Day mortality | p value | 60-Day mortality | p value | 90-Day mortality | p value | |||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | ||||
| Univariate analysis | |||||||||
| Sex, female9/25 (36%) | 4 | 5 | p=0.04OR=0.08 (0.01–0.93) | 4 | 5 | p=0.14 | 5 | 4 | p=0.09 |
| Steroid use17/25 (68%) | 5 | 12 | p=0.14 | 6 | 11 | p=0.13 | 8 | 9 | p=0.026OR=15.21 (0.76–305.3) |
| Mechanical ventilation16/25 (64%) | 5 | 11 | p=0.12 | 6 | 10 | p=0.056 | 8 | 8 | p=0.02 |
| Length of stay, days44 (21–96) | 21 (13.5–33) | 70 (111.5–22.25) | p=0.024 | 25 (16.25–53) | 69 (22–115) | p=0.11 | 33 (72–19.5) | 45 (119.5–21.5) | p=0.27 |
| Stratified subanalysis | |||||||||
| Sex+steroids:Female+steroids6/25 (24%) | 4 | 2 | p=0.011 | 4 | 2 | p=0.033 | 5 | 1 | p=0.011 |
| Sex+HIV:Female+HIV non-infected8/25 (32%) | 4 | 4 | p=0.048OR=0.08 (0.01–0.98) | 4 | 4 | p=0.15 | 5 | 3 | p=0.16 |
| Sex+steroids+HIV:Female+steroids+HIV non-infected6/25 (24%) | 4 | 2 | p=0.052 | 4 | 2 | p=0.12 | 5 | 1 | p=0.048OR=0.08 (0.01–0.94) |
Abbreviations: HIV, human immunodeficiency virus.
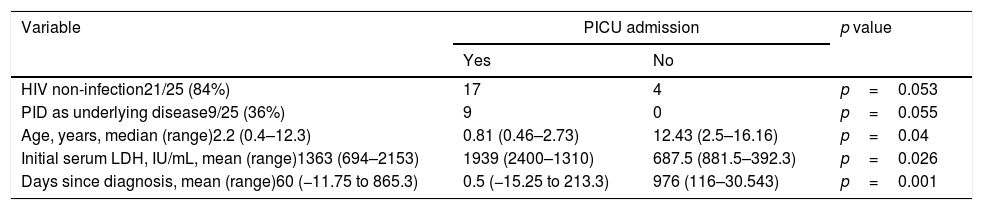
Variables associated with PICU admission were younger age, shorter time interval between the diagnosis of PJP and the diagnosis of the underlying disease, and higher lactate dehydrogenase (LDH) values. Only marginally significant differences in PICU admission were found between HIV non-infected and HIV-infected patients, and between PID and non-PID patients (Table 3).
Prevalence of significant (and marginally significant) risk factors and variables associated with PICU admission.
| Variable | PICU admission | p value | |
|---|---|---|---|
| Yes | No | ||
| HIV non-infection21/25 (84%) | 17 | 4 | p=0.053 |
| PID as underlying disease9/25 (36%) | 9 | 0 | p=0.055 |
| Age, years, median (range)2.2 (0.4–12.3) | 0.81 (0.46–2.73) | 12.43 (2.5–16.16) | p=0.04 |
| Initial serum LDH, IU/mL, mean (range)1363 (694–2153) | 1939 (2400–1310) | 687.5 (881.5–392.3) | p=0.026 |
| Days since diagnosis, mean (range)60 (−11.75 to 865.3) | 0.5 (−15.25 to 213.3) | 976 (116–30.543) | p=0.001 |
Abbreviations: HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; PID, primary immunodeficiency; PICU, pediatric intensive care unit.
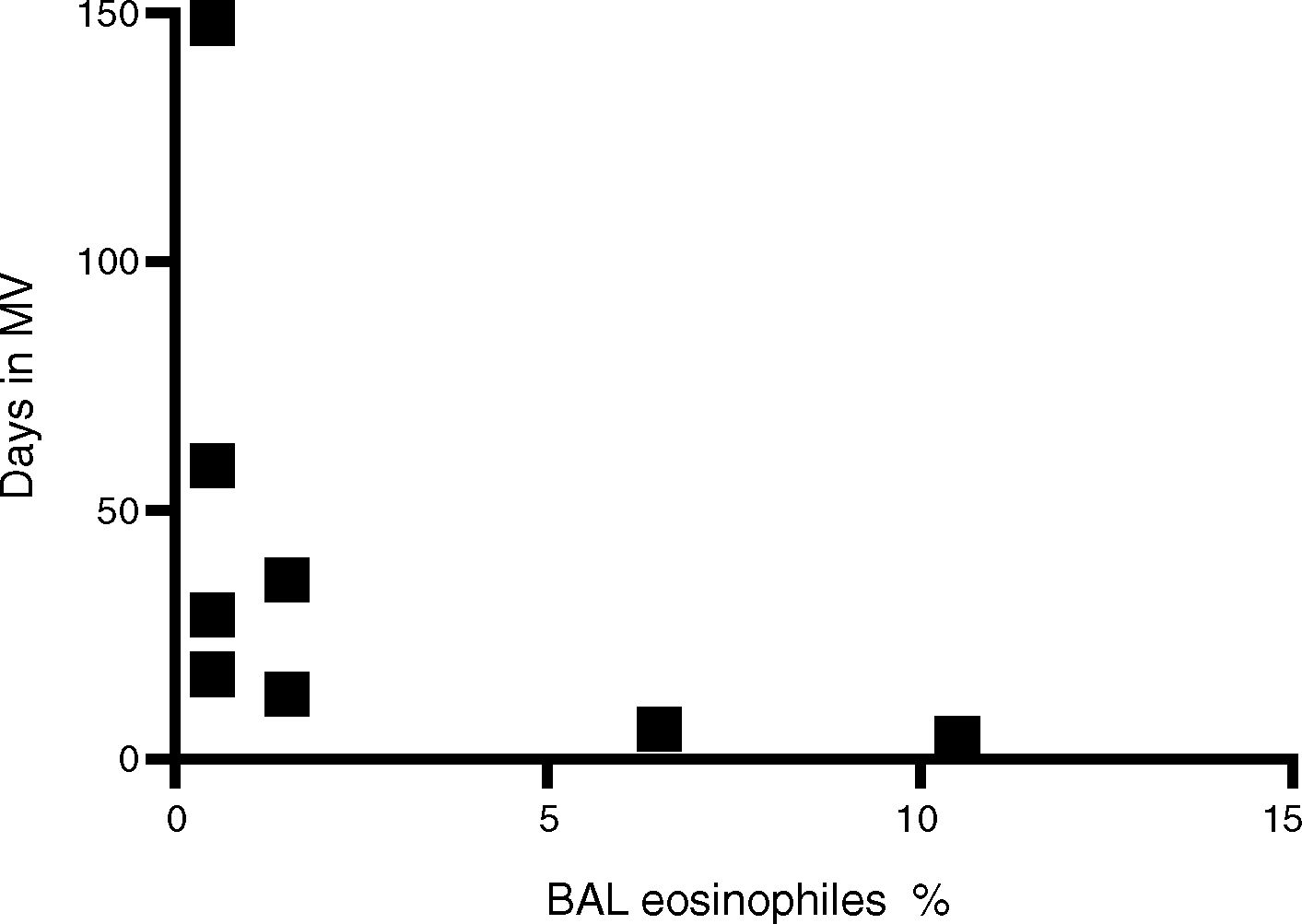
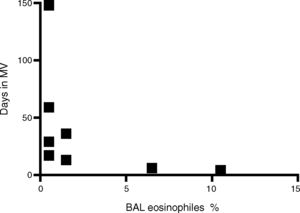
Finally, there was a negative correlation between percent eosinophil count in BAL and both length of PICU stay and duration of mechanical ventilation (r=−0.779 p<0.05) (Fig. 2).
DiscussionOur study includes one of the largest European cohorts of children with PJP and shows that after the HAART-era, Pneumocystis jirovecii pneumonia remains a life-threatening condition in children with a high risk of PICU admission and a high mortality rate, especially in patients with primary immunodeficiencies. Female gender, the use of steroids and HIV non-infected children are the main predictors of poorer prognosis.
Previous retrospective pediatric studies in the pre-AIDS era focused mainly on malignant diseases and PIDs as the underlying conditions and reported mortality rates of 32–56%,21,22 the lower end being similar to that of the present study.
More recent studies described the outcome of PJP in the HIV-infected pediatric population and highlighted the importance of CMV coinfection. Gibb et al. described 24 PJP cases in infants born in the United Kingdom and described a mortality rate of 63%. A second pathogen was detected in almost half the cases, with CMV predominating.23 Cooper et al. focused on PICU admissions during a 10-year period and documented 26 PJP cases; the authors reported CMV coinfection in more than 75% and an overall mortality rate of 27%.24 A third study specifically addressed the potential role of CMV as a coinfective agent in PJP and showed that coinfected patients were significantly more likely to be ventilated, undergo high-frequency oscillatory ventilation, and receive steroids; however, this last factor was not associated with increased survival.25 In contrast, although a significant rate of coinfection (26%) was observed in our study, it was not statistically associated with a poorer clinical outcome, likely because of the low prevalence of HIV-infected patients or insufficient statistical power to detect these differences.
Of note, the 2 cases of PJP in patients who underwent solid organ transplantation occurred when prophylaxis had been discontinued based on international consensus recommendations (107 days after kidney transplantation and 472 days after heart transplantation, respectively). In this sense, certain studies highlighting the risk of PJP several years after transplantation have suggested the need for more prolonged prophylaxis.26,27
Regarding differences in mortality according to HIV infection, a retrospective study in adults reported a mortality rate of 34% in HIV non-infected patients.28 As occurred in our cohort, a prospective French study with 544 cases of PJP reported greater mechanical ventilation requirement, and subsequent higher mortality in HIV non-infected than HIV-infected patients (mortality 27% vs 4% p<0.01). In this study, PJP treatment was started later in non-AIDS patients than in AIDS patients, and this delay was independently associated with higher mortality. Treatment initiation differed by only 1 day for AIDS versus non-AIDS patients, however, this difference was not seen in our regarding any of the outcome measures.29 A 10-year retrospective study in adults performed in the Unites States also reported significant differences in ICU admissions, need for mechanical ventilation, and mortality in relation to HIV infection (HIV-infected 9.6% vs HIV non-infected 39.4%).30 A very similar study to ours, focused on an 80-children cohort, revealed a mortality of 35.4% in HIV-infected patients, higher than in HIV non-infected population (25.5%, the difference not being statistically significant), just the opposite to our results and the ones previously reported on adults. It must be stated that in this study 55% of deceased HIV-infected children had important comorbidities. Three of these patients did not present any underlying immunological disorder.31
One hypothesis to explain these differences in prognosis could be a differing burden of PJ and differing neutrophil and eosinophil counts in lung between the two populations: one study showed that the HIV non-infected population has a lower PJ burden and higher neutrophil count in BAL, with this latter factor related to poorer gas exchange performance.32 Regarding eosinophil count in BAL, Fleury-Feith et al. reported that patients with AIDS-related PJP, which had a better prognosis than AIDS-unrelated cases, showed higher eosinophil counts in BAL specimens.33 In our cohort it was not possible to compare the neutrophil and eosinophil counts between HIV-infected and HIV non-infected patients since only 1 out of 12 available BAL samples came from HIV-infected patients. However, when globally assessing the role of different BAL cell populations, only a lower count of eosinophils was associated with longer PICU stay and need of mechanical ventilation. The role of eosinophils in promoting the clearance of PJ has been elucidated by analyzing the eosinophils signature after RNA-sequencing of whole lung in the murine model. Higher counts of eosinophils were obtained in wild type mice than in CD4+ T-cells depleted ones. Also, in SCID and CD4+ T-cells mice treated with IL-5 to promote eosinophilia in the lung resulted in a lower PJ burden compared with their respective controls and with eosinophilopoiesis-deficient GATA1 mice.34
Although there are fewer data in the HIV non-infected population, two recently published studies focused on pediatric patients without HIV infection. Kim et al. analyzed 15 cases occurring over a 14-year period (2000–2014) and reported a 60-day mortality rate of 35.7% which is somewhat higher than in our HIV non-infected population (28.6%). To note, only 13.3% of them were receiving active prophylaxis within 1 month before the PJP episode.35 The second study collected 60 cases of PJP over a 10-year period (2005–2014).36 Overall mortality was 41.66%, and PICU admission rate was 55%. In total, 43.3% of patients had coinfections, and higher LDH levels were significantly associated with a poorer prognosis. Similar conclusions have been seen in an adult retrospective study that spanned 17 years (2000–2017), where LDH was a predictor of in-hospital mortality. Furthermore, LDH levels over 496iU/L were associated with higher mortality than overall population.37 In the present study, LDH levels correlated with a higher rate of PICU admissions and the need for mechanical ventilation, but not with overall mortality.
In our cohort, we found two patients without underlying immunological disorders. As for dihydrolipoamide dehydrogenase deficiency, lipoate metabolism has been described in bacterial, fungal and protozoan organisms, affecting their virulence and pathogenesis, although deficiency of any of these enzymes has not been associated with PJP in humans.38 We have not found previously published reports or a plausible explanation for PJP in patients with progressive familial intrahepatic cholestasis type II prior to liver transplantation.
With regard to steroid administration as adjunctive therapy, steroid use has proven to be more effective than antimicrobial therapy alone for severe PJP in the adult HIV-infected population, but it remains controversial in children39 and in HIV non-infected patients.40 In accordance with this concern, we did not find a positive effect of steroids in our cohort, probably because they were used in more severe cases.
The limitations of this study are its retrospective nature and the relatively small sample size. Although our sample is the largest reported in our country and one of the largest pediatric studies in the recent years, the statistical power to detect differences is likely limited.
To conclude, PJP is still persisting as a life-threatening condition in immunocompromised children, particularly in HIV non-infected patients. The decrease in the incidence of vertically transmitted HIV infection, the advances in the diagnosis of PID, and the increasing use of a wide range of immunocompromising therapies, such as transplantation and chemotherapy for cancer have shifted the focus of conditions associated with PJP. Clinicians should have a high index of suspicion of PJP in patients affected by these conditions. On the other hand, the discovery of PJP in a child who has not been diagnosed of an underlying disease should always lead to a thorough evaluation of the immune system. Additionally, the persistent, undesirable PJP mortality rate should encourage us to carry out randomized controlled pediatric trials to elucidate questions that remain unanswered, such as the influence of adjuvant steroids in the treatment of severe PJP in the HIV non-infected population, the role of percent eosinophils in BAL specimens in terms of prognosis, and the benefits of more prolonged prophylaxis against PJP in children undergoing solid organ transplantation, particularly in those receiving high dose immunosuppressive therapy. Finally, it should not be forgotten that adequate prophylaxis is the best strategy to prevent PJP in patients with immunocompromising conditions.
FundingNo external funding has been received for this manuscript.
Conflict of interestThe authors have neither financial relationships nor conflicts of interest relevant to this article to disclose.
We thank Beatriz Bistuer-Salamero for her inspiring idea and Celine Cavallo for English language support.