A 52-year-old woman who was admitted to the pulmonology department with a diagnosis of pneumonia which had started 10 days prior to admission, and with an unfavourable clinical course, despite antibiotic treatment with cefditoren and azithromycin. Her medical history referred to right-sided pneumonia in her youth. She has a sedentary lifestyle and works as an economist. She attended because of a non-productive cough, fever and soft stools. Bronchial breath sounds, hypophonesis and moist crackling could be heard in the base of the right lung during the pulmonary auscultation. The rest of the examination was normal.
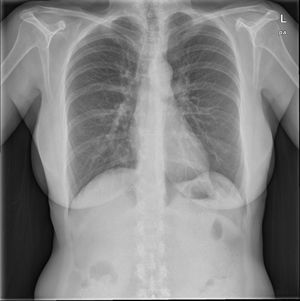
The lab tests revealed: red blood cells 368,000; haemoglobin 11.9; Hct 36.6; MCV 99.4; leukocytes 21,800 (88°N); platelets 414,000 and CRP 150; normal biochemistry and urine with no abnormalities. The chest X-ray revealed poorly defined alveolar consolidation in the right lower lobe (RLL). There was no pleural effusion. Complete immunological testing, including immunoglobulins G, A and M, rheumatoid factor and anti-cellular and anti-neutrophil cytoplasmic antibodies, was normal. Microbiological testing of sputum was negative, including smears and fungal tests. Legionella antigen in urine, as well as serological tests for Coxiella burnetii, Mycoplasma pneumoniae, Chlamydia, Brucella, Epstein–Barr virus, varicella-zoster virus, hepatitis A, B and C viruses, human immunodeficiency virus, toxoplasma, herpes virus, CMV, stool cultures and Clostridium difficile toxin, were all negative.
Treatment was started with piperacillin/tazobactam and levofloxacin with limited response. Irregular fever (up to 38.5°C), diarrhoea, nausea and general malaise were maintained. It was therefore decided to suspend the established empirical treatment. The suspension of treatment was accompanied by disappearance of the fever and improvement of the patient's general condition. However, she still had a non-productive cough and physical signs of parenchymal involvement, with the onset of crackling in both lungs. A new chest X-ray revealed consolidation in both lower lobes (LL). An echocardiographic study was performed, which was within normal limits.
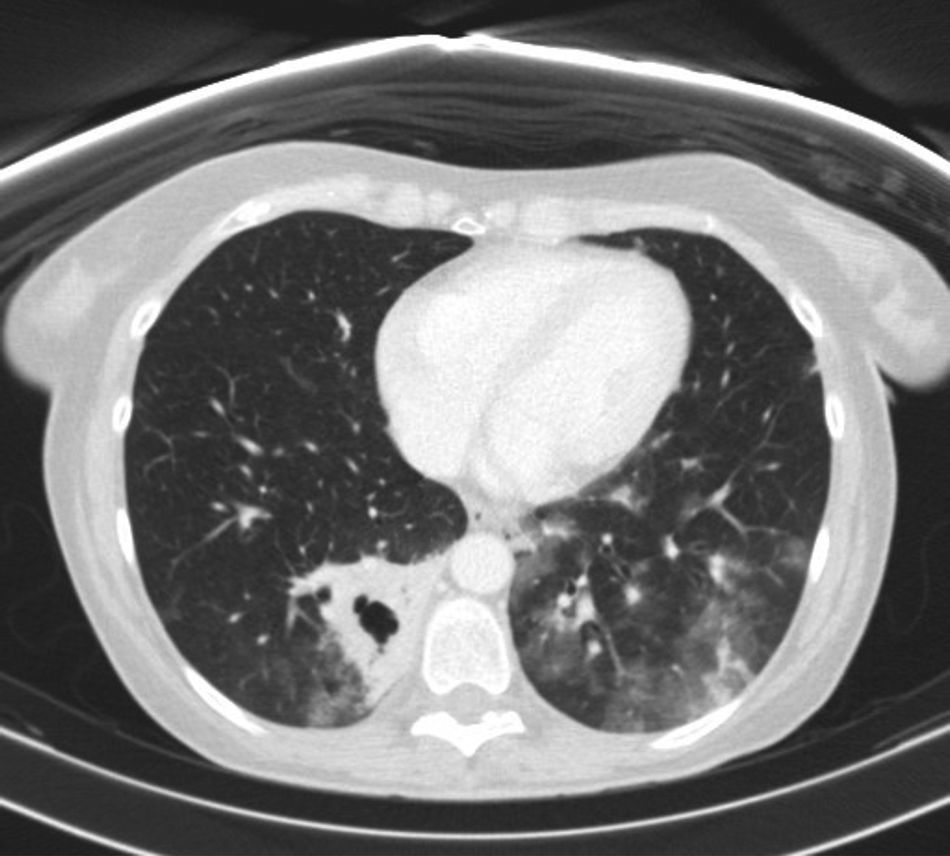
The computed tomography (CT) (Fig. 1) showed a cavitated consolidation in the RLL with extensive areas of ground-glass opacity in both LLs. These findings suggest, as a first possibility, an infectious cause with cavitation and bronchogenic spread (Fig. 2). The Mantoux intradermal reaction test was negative. The galactomannan in blood test was negative.
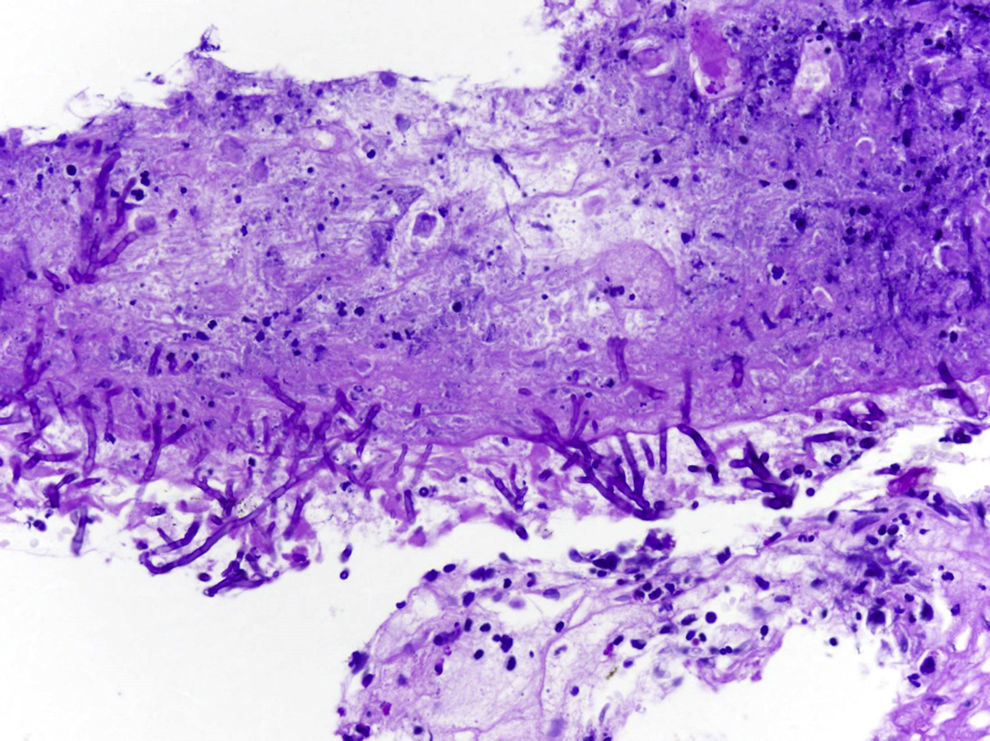
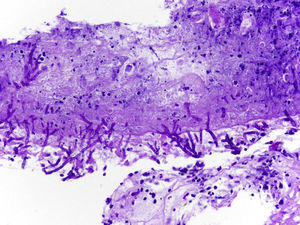
Fibrobronchoscopy (FB) was performed, which showed signs of inflammation at the entry to the posterior segmental bronchus of the upper lobe of the right lung. The transbronchial biopsy (TBB) revealed necrotic mucosa with the presence of fungal structures suggestive of Aspergillus (Fig. 2). Growth of Aspergillus fumigatus complex was observed in the bronchial suction, bronchoalveolar lavage and telescoped brushed material of the RLL.
Clinical courseTreatment was started with voriconazole and micafungin according to the protocol of the 2016 Infectious Diseases Society of America (IDSA) guidelines.1 When abdominal discomfort and vomiting developed with the switch to oral therapy, it was replaced by itraconazole. Treatment with micafungin was adhered to for 10 days.
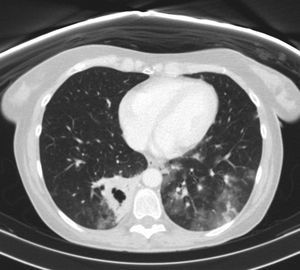
After six months of treatment with itraconazole, the patient remained asymptomatic and with normalisation of the lab tests, but with persistence of a small nodular lesion in the RLL (Fig. 3).
CommentsVarious risk factors for the development of invasive pulmonary aspergillosis (IPA) are recognised. Most of them are immunodeficiencies and they are rare in healthy patients. Symptoms are not very specific in neutropaenic patients or in immunocompetent patients. They include the unfavourable progression of pneumonia with fever and non-productive cough.2,3
The incidence of IPA in patients with no classic risk factors has increased in recent years. Radowsky et al.4 report a co-infection with Mucor in a healthy 22-year-old male. There are other cases in immunocompetent patients with concomitant diseases. Olaechea Astigarraga et al.5 carried out a review of non-immunosuppressed cases associated with chronic obstructive pulmonary disease and cirrhosis which were admitted to intensive care. Moreno-González et al.6 presented the case of a woman with disease spread by A. fumigatus and Aspergillus flavus, and a history of kidney disease.
We believe that our case is rare as it concerns a healthy woman with IPA with no relevant or subsequently observed medical history. Due to the persistence of symptoms, radiological progression and the absence of a specific diagnosis, after some non-conclusive lab tests and radiological results, we resorted to fibrobronchoscopy, which revealed the presence of A. fumigatus in the transbronchial biopsy.7–9
Although the 2016 IDSA guidelines1 do not recommend the use of combined initial therapy (voriconazole and echinocandin), its use is permitted in selected cases. Given the rarity of our case, we decided upon initial treatment with intravenous voriconazole for 15 days, checking therapeutic levels, and micafungin for 10 days. Subsequently, in light of the poor tolerance to oral voriconazole, we changed to itraconazole. We rejected posaconazole as the patient was immunocompetent and tolerated itraconazole well, in light of the good clinical response, considering that both antifungals demonstrated similar clinical evidence as rescue treatments.10
Please cite this article as: Sánchez-Jareño M, Martínez Verdasco A, Esteban Rodriguez I, Álvarez-Sala R. Neumonía con evolución inusual en paciente inmunocompetente. Enferm Infecc Microbiol Clin. 2018;36:382–383.