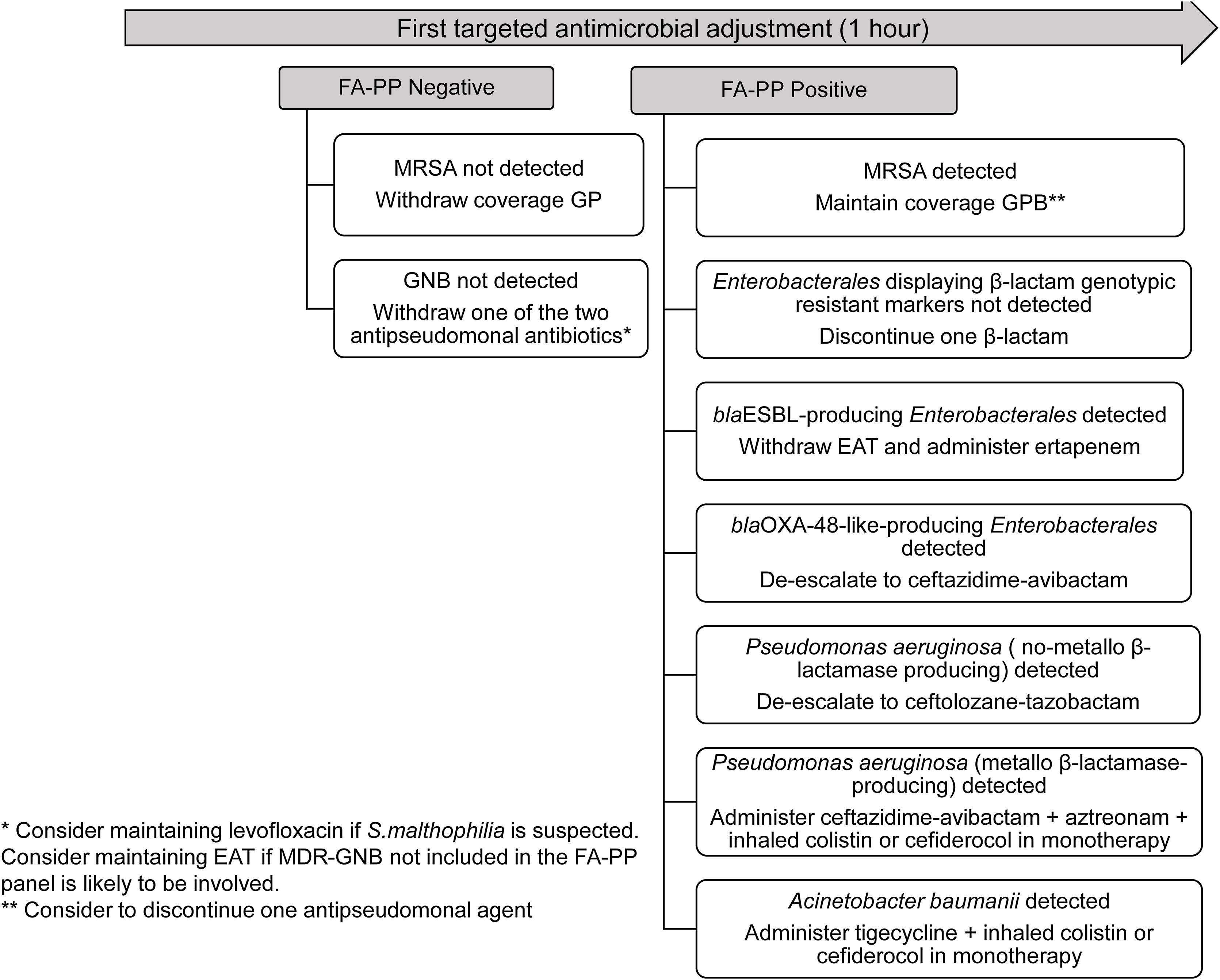
Ventilator-associated lower respiratory tract bacterial infection (VA-LRTBI) is associated with high morbidity and mortality, notably when multidrug-resistant bacteria (MDRB) are involved and empirical antimicrobial therapy (EAT) is inadequate.1,2 Conventional semiquantitative culture-based antimicrobial susceptibility testing (AST) procedures performed on lower respiratory tract specimens return results approximately 48–72h after specimen receipt. The BioFire® FilmArray® Pneumonia/Pneumonia plus Panel (FA-PP) (BioFire Diagnostics, LLC, Salt Lake City, UT) is a multiplex PCR panel that, in addition to respiratory viruses and “atypical” bacteria, tests for several bacteria commonly involved in VA-LRTBI (yielding semiquantitative estimates of bacterial loads) and seven genetic antibiotic resistance markers (mecA/C and MREJ, blaCTX-M, blaKPC, blaVIM, blaOXA-48-like, blaIMP, and blaNDM); its use may provide clinically actionable results within about an hour of specimen reception. We and others previously demonstrated that FA-PP notably increases the diagnostic yield in LRTBI and narrows the time window for results compared with culture-based methods; this may prompt early EAT adjustment, which could be cost-beneficial by decreasing antimicrobial use and shortening the intensive care unit (ICU) stay.3,5,8,9 Nevertheless, consensus criteria for antimicrobial stewardship according to FA-PP results need to be established. Here, we present an actionable antimicrobial stewardship algorithm (Fig. 1) for early EAT adjustment in patients with suspicion of VA-LRTBI at the ICU of the Hospital Clínico Universitario of Valencia, based upon the FA-PP results, planned to be formally evaluated beginning June 2023 upon approval by the INCLIVA (Instituto de Investigación Sanitaria, Hospital Clínico Universitario) Ethics Committee. The algorithm was built based on several assumptions mainly derived from the literature available regarding FA-PP's analytical and clinical performances3–9: (i) the high negative predictive value of the assay for all targets in the panel; (ii) the potential etiological relevance of all detectable bacteria at any load (limit of detection: ≥103.5log10/ml; limit of quantification, 104log10copies/ml) in patients undergoing EAT; (iii) the frequent involvement of lower respiratory tract-colonizing MDRB in VA-LRTBI; (iv) bacteria other than those targeted by the FA-PP panel could be involved in VA-LRTBI (i.e. some Enterobacterales species and Stenotrophomonas maltophilia). As per protocol, based on consensus guidelines10 and local epidemiology, a combination of two antimicrobials displaying antipseudomonal activity, including a beta-lactam/beta-lactamase inhibitor (such as ceftolozane/tazobactam, ceftazidime/avibactam, meropenem or piperacillin/tazobactam) plus amikacin, quinolone or inhaled colistin, and an additional drug covering methicillin-resistant Staphylococcus aureus (MRSA) (mainly linezolid) is used as EAT for VA-LRTBI at our center. Proposed EAT adjustments based on FA-PP results are the following: (i) withdraw antimicrobial coverage of Gram-positive bacteria if MRSA is not detected; (ii) withdraw one of the two antipseudomonal agents if Gram-negative bacteria are not detected. In this scenario, quinolone therapy might be maintained if S. maltophilia is potentially relevant according to local epidemiology; (iii) maintain coverage of Gram-positive bacteria if MRSA is detected and consider discontinuing one antipseudomonal agent; (iv) discontinue one antipseudomonal agent if Enterobacterales with no genotypic resistant trait are detected; (v) withdraw EAT and administer ertapenem when extended spectrum β-lactamase-(ESBL)-producing Enterobacterales are detected; (vi) de-escalate to ceftazidime-avibactam if blaOXA-48-like-producing Enterobacterales is detected; (vii) de-escalate to ceftolozane-tazobactam if non-metallo-β-lactamase-producing Pseudomonas aeruginosa is detected; (viii) upon detection of metallo-β-lactamase-producing P. aeruginosa, administer ceftazidime-avibactam and aztreonam and inhaled colistin or cefiderocol in monotherapy; (ix) detection of Acinetobacter baumanii should prompt the administration of tigecycline and inhaled colistin or cefiderocol in monotherapy; (x) add oseltamivir if influenza virus is detected.
Early adjustment of empirical antimicrobial therapy in patients with suspicion of ventilator-associated lower respiratory tract bacterial infection based on the BioFire® FilmArray® Pneumonia/Pneumonia plus Panel (FA-PP) results. The following microorganisms are targeted by FA-PP: Acinetobacter calcoaceticus-baumannii complex, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella aerogenes, Klebsiella oxytoca, Klebsiella pneumoniae group, Moraxella catarrhalis, Proteus spp., Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes, Chlamydia pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, Adenovirus, Coronavirus, Human metapneumovirus, Human rhinovirus/enterovirus, Influenza A virus, Influenza B virus, Parainfluenza virus, and Respiratory syncytial virus. EAT, empirical antimicrobial therapy; ESBL, extended spectrum β-lactamase; GNB, Gram-negative bacteria; GPB, Gram-positive bacteria; MRSA, methicillin-resistant Staphylococcus aureus.
Several considerations related to the above: (i) in all the above scenarios, consider maintaining primary EAT regardless of FA-PP results when patients are severely immunosuppressed, present with septic shock, an additional infection source is suspected or MDR bacterial species not included in the panel are likely to be involved according to patient's risk factors or local epidemiology; (ii) detection of two or more Gram-negative bacterial targets harboring genotypic resistant traits (if particular fermenting and non-fermenting bacteria are detected in combination) will require individualized antimicrobial therapy tailoring; (iii) the algorithm has been designed taking into consideration the bacterial epidemiology at our ICU, which may not be extrapolatable to other settings.
The suitability of ongoing antimicrobial therapy (either adjusted or not upon FA-PP results) in terms of coverage as well as treatment duration should be re-assessed within 48h, following receipt of microbiological results from standard semiquantitative cultures and conventional AST, taking into consideration the patient's clinical status. A concluding remark: this is a list of some of the many potential actions in terms of EAT adjustments that may be derived from FA-PP results. We naturally open our proposal for discussion, which we are confident will translate into tangible benefits for our ICU patients.
Authors’ contributionsMAC, NC, EA and DN designed the algorithm and wrote the manuscript.
FundingThe authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interestThe authors have no relevant competing interest to disclose in relation to this work.
We are grateful to Josep Ferrer and ICU staff for their contribution to the present work.