Skin and soft-tissue infections (SSTIs) are common and are linked to a wide variety of clinical conditions. Few studies have analysed the factors associated with mortality and re-admissions in medical patients with SSTIs. Accordingly, this study sought to describe the clinical and microbiological characteristics of patients diagnosed with SSTIs, and identify mortality and re-admission related factors.
Patients and methodsA total of 308 patients were included in the study. Clinical, socio-demographic and microbiological characteristics were collected. Univariate and logistic regression multivariate analyses were performed in order to identify factors associated with mortality and re-admission.
ResultsThe bacteria responsible were identified in 95 (30.8%) patients, with gram-positive bacteria being isolated in 67.4% and gram-negative in 55.8% of cases. Multi-resistant bacteria were frequent (39%), and the initial empirical treatment proved inadequate in 25.3% of all cases. In-hospital mortality was 14.9%; the related variables were heart failure (OR=5.96; 95%CI: 1.93–18.47), chronic renal disease (OR=6.04; 95%CI: 1.80–20.22), necrotic infection (OR=4.33; 95%CI: 1.26–14.95), and inadequate empirical treatment (OR=44.74; 95%CI: 5.40–370.73). Six-month mortality was 8%, with the main related factors being chronic renal disease (OR: 3.03; 95%CI: 1.06–8.66), and a Barthel Index score of under 20 (OR: 3.62; 95%CI: 1.17–11.21). Re-admission was necessary in 26.3% of cases, with the readmission-related variables being male gender (OR: 2.12; 95%CI: 1.14–3.94), peripheral vascular disease (OR: 3.05; 95%CI: 1.25–7.41), and an age-adjusted Charlson Comorbidity Index score of over 3 (OR: 3.27; 95%CI: 1.40–7.63).
ConclusionsClinical variables such as heart failure, chronic renal disease, peripheral vascular disease, and necrotic infection could help identify high-risk patients. The main factor associated with higher mortality was inadequate initial empirical treatment. Physicians should consider gram-negative, and even extended-spectrum beta-lactamase-producing bacteria when assigning initial empirical treatment for SSTIs, especially in healthcare-associated cases.
Las infecciones de piel y partes blandas (IPPB) son frecuentes y se asocian a una amplia variedad de presentaciones clínicas. Los factores asociados a mortalidad y reingreso en pacientes con IPPB han sido poco estudiados hasta ahora. En este sentido, el objetivo del presente trabajo es describir las características clínicas y microbiológicas de pacientes diagnosticados de IPPB e identificar factores asociados a mortalidad y reingreso en ellos.
Pacientes y métodosFueron incluidos un total de 308 pacientes. Se realizó una descripción de las características clínicas, sociodemográficas y microbiológicas. Se llevaron a cabo análisis uni y multivariantes de regresión logística para identificar factores asociados a mortalidad y reingreso en pacientes con IPPB.
ResultadosLos microorganismos responsables fueron identificados en 95 (30,8%) pacientes, de ellos el 67,4% presentaban bacterias grampositivas y el 55,8%, gramnegativas. La presencia de bacterias multirresistentes fue frecuente (39%) y el tratamiento empírico fue inadecuado en el 25,3% de los casos. La mortalidad intrahospitalaria fue del 14,9% y las variables asociadas a ella fueron la insuficiencia cardiaca (OR=5,96; IC95%: 1,93-18,47), la insuficiencia renal crónica (OR=6,04; IC95%: 1,80-20,22), la infección necrótica (OR=4,33; IC95%: 1,26-14,95) y el tratamiento antibiótico empírico inadecuado (OR=44,74; IC95%: 5,40-370,73). La mortalidad a 6 meses fue del 8%, y los principales factores asociados, la insuficiencia renal crónica (OR=3,03; IC95%: 1,06-8,66) y una puntuación en el índice de Barthel inferior a 20 puntos (OR=3,62; IC95%: 1,17-11,21). Reingresaron durante el seguimiento a 6meses el 26,3% de los pacientes; las variables asociadas a este hecho fueron el sexo masculino (OR=2,12; IC95%: 1,14-3,94), la enfermedad vascular periférica (OR=3,05; IC95%: 1,25-7,41) y una puntuación en el índice de Charlson ajustado por edad superior a 3puntos (OR=3,27; IC95%: 1,40-7,63).
ConclusionesVariables clínicas como la insuficiencia cardiaca, la insuficiencia renal crónica, la enfermedad vascular periférica y la infección necrótica podrían ayudar a identificar pacientes con IPPB de alto riesgo. El principal factor asociado a una mayor mortalidad fue el tratamiento antibiótico empírico inadecuado. Debería considerarse la posibilidad de que bacterias gramnegativas, o incluso enterobacterias productoras de betalactamasas de espectro extendido, sean las responsables de IPPB, sobre todo en casos asociados a los cuidados sanitarios, a la hora de plantear el tratamiento antibiótico empírico en estos pacientes.
Skin and soft-tissue infections (SSTIs) include a wide variety of epidermis, dermis, subcutaneous tissue and muscle infections. They commonly present with a broad spectrum of clinical manifestations, ranging from mild to life-threatening forms.1 Gram-positive microorganisms, and Staphylococcus aureus in particular, usually cause SSTIs, though gram-negative bacteria and anaerobes may also be implicated.2,3 In most cases, the specific pathogen responsible cannot be identified, due to the low efficiency of microbiological cultures.4 These diseases are commonly diagnosed in emergency wards5 as well as among hospitalised1,6 and critically ill patients,7 with incidence being seen to rise in recent years.6
It is essential to consider the clinical characteristics of these patients (diabetes mellitus, obesity, vascular disease, traumatism, recent surgery) and/or the possible existence of immunocompromised status [human immunodeficiency virus infection, immunosuppressive therapy, advanced age], because of the potential role that these factors may play in predisposition to SSTI development and worse disease progression.4 In this regard, there are few studies that describe clinical factors linked to SSTI development, and fewer still in the case of variables associated with higher mortality and readmission.1,8 Moreover, most of previous series describe cases from both medical and surgical departments, whose characteristics could be quite different.1,8
The dual aim of this study was thus: to analyse the epidemiological, clinical, analytical and microbiological characteristics potentially linked to SSTIs in medical patients; and to identify the factors associated with higher mortality and readmission.
Patients and methodsWe conducted a retrospective analysis which covered all patients diagnosed with SSTIs at the Internal Medicine Department of the Santiago de Compostela University Teaching Hospital (Complejo Hospitalario Universitario de Santiago) (NW Spain), from 1 October 2010 to 31 December 2013, as shown by the hospital discharge database. This search included all patients identified with the following ICD 10 (International Classification of Diseases, 10th edition) codes: 680.*; 681.*; 682.*; 683.*; 686.*; 035.* and 785.*. The diagnosis criteria followed to include patients and classify them into the different type of SSTIs were those published in current guidelines.9
Through patients’ medical histories review, we first exclude all patients who did not met SSTIS criteria.9 After that, we recorded their socio-demographic, clinical and analytical characteristics, and then followed them up for six months post-discharge. We evaluated and quantified case complexity using the age-adjusted Charlson Comorbidity Index (CCI),10 and established patients’ grade of physical dependence using the Barthel Index (BI).11
Cases were classified as follows: nosocomial, where diagnosis had been established later than the second day after hospital admission; and healthcare-associated, in those instances where they had come from nursing homes, been hospitalised, or been treated with intravenous antibiotics during the 90 previous days. All other cases were identified as community-acquired.
We considered as fever a corporal temperature higher than 37.8°C and sepsis was defined according to the Surviving Sepsis Campaign criteria, current during the study period.12 Empirical antibiotic treatment was deemed inadequate in the following cases: if the first administered antibiotic proved ineffective for the isolated bacteria after culture (microbiological criteria); or if it was changed due to therapeutic failure during the first 72h, based on clinical criteria.
All patients deceased during their hospitalisation were included as in-hospital mortality. A 6-month follow up was made in all survivors; in those cases mortality and readmission by any cause were recorded and also specific SSTIs-related mortality and readmission.
A descriptive analysis was performed, by calculating qualitative-variable rates plus mean and standard deviation. We used the Chi-square test or Fisher's exact test, as appropriate (expected frequency value <5), to compare qualitative variables, and the Student's t test for quantitative variables. A multivariate logistic regression analysis was conducted to identify factors associated with mortality and readmission. Akaike's information criterion (AIC), which combines the goodness of fit with the number of parameters, was used to select the best model.13 The model with the lowest AIC value was considered to have the best fit. A P-value <0.05 was regarded as significant. All analyses were performed using the SPSS v. 22.0 software package (SPSS Inc., Chicago, IL, USA).
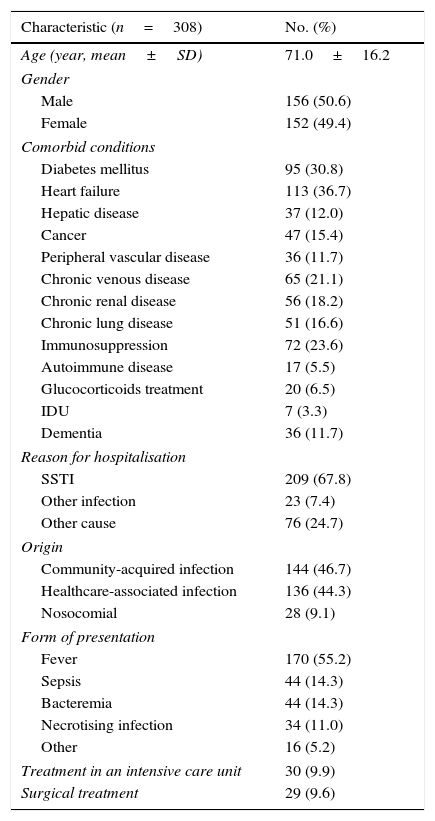
ResultsDuring the study period, there was a total of 308 patients with SSTIs, 50.6% men, mean age 71.3 years (standard deviation [SD]=16.2). In 11.2% of cases, BI scores were less than 20, indicating a severe degree of physical dependence. All patients’ histories could be reviewed and 6-month follow up was fulfilled in all cases. The median of age-adjusted CCI score was 5.5 (IQR=3) and 172 (56%) patients had 2 or more chronic diseases. The majority of patients (68.2%) lived at home, with the result that most of the cases (47%) were classified as community-acquired, followed by healthcare-associated (44%) and nosocomial (9%). The main comorbid conditions were heart failure (36.7%), diabetes mellitus (30.8%) and immunosuppression (23.6%). Other socio-demographic and overall clinical characteristics are shown in Table 1.
Clinical and epidemiological characteristics of the 308 included patients.
| Characteristic (n=308) | No. (%) |
|---|---|
| Age (year, mean±SD) | 71.0±16.2 |
| Gender | |
| Male | 156 (50.6) |
| Female | 152 (49.4) |
| Comorbid conditions | |
| Diabetes mellitus | 95 (30.8) |
| Heart failure | 113 (36.7) |
| Hepatic disease | 37 (12.0) |
| Cancer | 47 (15.4) |
| Peripheral vascular disease | 36 (11.7) |
| Chronic venous disease | 65 (21.1) |
| Chronic renal disease | 56 (18.2) |
| Chronic lung disease | 51 (16.6) |
| Immunosuppression | 72 (23.6) |
| Autoimmune disease | 17 (5.5) |
| Glucocorticoids treatment | 20 (6.5) |
| IDU | 7 (3.3) |
| Dementia | 36 (11.7) |
| Reason for hospitalisation | |
| SSTI | 209 (67.8) |
| Other infection | 23 (7.4) |
| Other cause | 76 (24.7) |
| Origin | |
| Community-acquired infection | 144 (46.7) |
| Healthcare-associated infection | 136 (44.3) |
| Nosocomial | 28 (9.1) |
| Form of presentation | |
| Fever | 170 (55.2) |
| Sepsis | 44 (14.3) |
| Bacteremia | 44 (14.3) |
| Necrotising infection | 34 (11.0) |
| Other | 16 (5.2) |
| Treatment in an intensive care unit | 30 (9.9) |
| Surgical treatment | 29 (9.6) |
SD: standard deviation. IDU: intravenous drug user. SSTI: skin and soft tissue infection.
Insofar as the localisation of infection was concerned, the point of entry was identified in 44.8% of cases. The lower extremities were the principal infection site (70.9%) followed by the upper extremities (7.8%). Cellulitis was the most frequent type of infection (70.8%), followed by abscesses (12.3%) and pressure ulcers (6.8%). Abscesses were in all cases complications from cellulitis or pyomyositis and pressure ulcers were located in sacral region and hips.
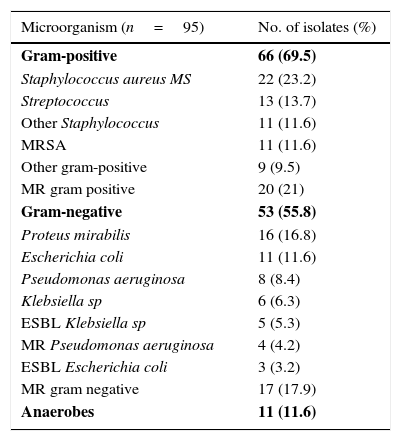
Sepsis criteria were identified in 39.9% (123 patients) and bacteraemia in 14.3% (44 patients) of cases, though blood cultures were drawn in 218 patients (70.8%). Of the 144 patients (46.8%) on whom local cultures were performed, 95 (30.8%) tested positive and were defined as patients with known responsible bacteria. Gram-positive bacteria were isolated in 64 (67.4%) and gram-negative in 53 cases (55.8%). Of these, 41 patients (43.2%) were infected exclusively by gram-positive bacteria and 24 (25.3%) exclusively by gram-negative bacteria; anaerobes were isolated in 11 cases (11.6%), all in combination with other types of bacteria. Two or more responsible bacteria (multi-bacterial origin) were identified in 46 (48.4%) cases. Multi-drug resistant (MR) gram-positive bacteria were isolated in 21% and MR gram-negative bacteria in 17.9% of patients with known responsible bacteria [8.4% extended-spectrum betalactamase (ESBL) producing bacteria]. The most common responsible organism was methicillin-sensible (MS) Staphylococcus aureus (23.2%), followed by Proteus mirabilis (16.8%). In healthcare-associated cases, the presence of gram-negative bacteria and multi-bacterial origin was much higher (69.4% and 51% respectively). A complete outline of the microbiological data is shown in Table 2. The etiological agent prevalence was similar in all types of SSTIs, without significant differences between them.
Microbiological profile and empirical antibiotic treatment.
| Microorganism (n=95) | No. of isolates (%) |
|---|---|
| Gram-positive | 66 (69.5) |
| Staphylococcus aureus MS | 22 (23.2) |
| Streptococcus | 13 (13.7) |
| Other Staphylococcus | 11 (11.6) |
| MRSA | 11 (11.6) |
| Other gram-positive | 9 (9.5) |
| MR gram positive | 20 (21) |
| Gram-negative | 53 (55.8) |
| Proteus mirabilis | 16 (16.8) |
| Escherichia coli | 11 (11.6) |
| Pseudomonas aeruginosa | 8 (8.4) |
| Klebsiella sp | 6 (6.3) |
| ESBL Klebsiella sp | 5 (5.3) |
| MR Pseudomonas aeruginosa | 4 (4.2) |
| ESBL Escherichia coli | 3 (3.2) |
| MR gram negative | 17 (17.9) |
| Anaerobes | 11 (11.6) |
| Antibiotic (n=308) | No. (%) |
|---|---|
| Amoxicillin plus clavulanate | 102 (33.1) |
| Cloxacillin | 80 (26.0) |
| Cephalosporins (2ª, 3ª or 4ª generation) | 45 (14.6) |
| Piperacillin plus tazobactam | 27 (8.8) |
| Ciprofloxacin | 22 (7.1) |
| Linezolid | 21 (6.8) |
| Clindamycin | 18 (5.8) |
| Levofloxacin | 12 (3.9) |
| Vancomycin | 9 (2.9) |
| Meropenem | 7 (2.3) |
| Metronidazol | 7 (2.3) |
| Teicoplanin | 6 (1.9) |
| Rifampicin | 5 (1.6) |
| Gentamicin | 3 (1.0) |
| Aztreonam | 3 (1.0) |
| Penicillin (V and G) | 2 (0.6) |
| Trimethoprim-sulfamethoxazole | 2 (0.6) |
| Amikacin | 2 (0.6) |
| Norfloxacin | 1 (0.3) |
MS: methicillin-sensible. MRSA: methicillin-resistant Staphylococcus aureus. MR: multi-drug resistant. ESBL: extended-spectrum beta-lactamases.
The most commonly used empirical antibiotic treatments were amoxicillin and clavulanate in 102 patients (33.1%) and cloxacillin in 80 patients (26.0%). In 91 (29.5%) cases a combination of antibiotics was used. The initial empirical treatment was inadequate in 78 cases (25.3%), 51 according to microbiological criteria and 27 according to clinical criteria. The factors associated with inadequate empirical treatment were peripheral vascular disease, surgery in the 90 days before current admission, nosocomial origin, and multi-bacterial infection (P<0.05).
In-hospital mortality was 14.9% (46 patients) and mortality across six-month follow-up was 8% (21 patients). Readmission was necessary in 69 (26.3%) cases (34 of them, 49%, caused by a SSTIs).
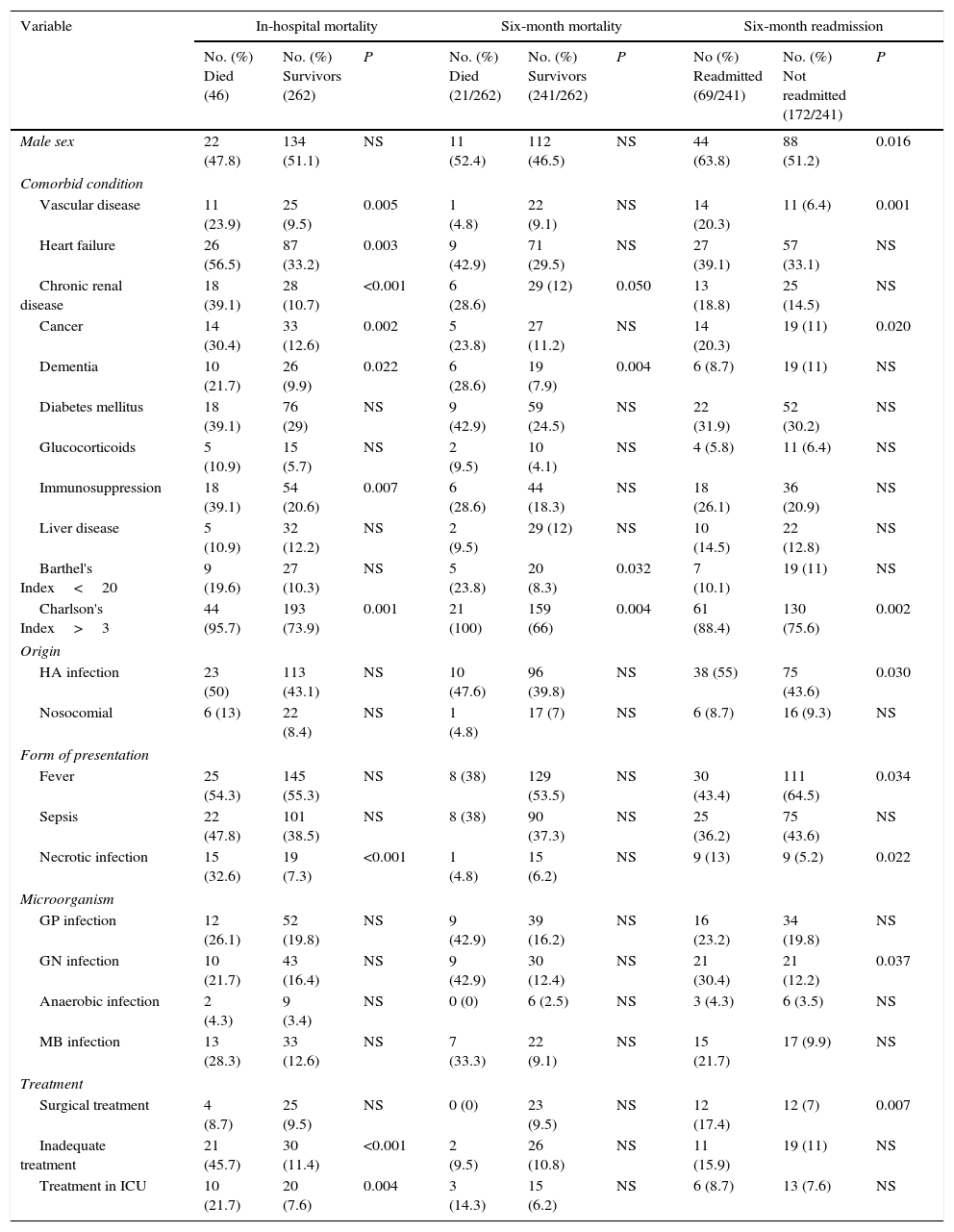
While the main factors shown by the univariate analysis (P<0.05) to be associated with in-hospital mortality, six-month mortality and six-month readmission are listed in Table 3. If we focus in readmission caused by SSTIs, the only variables associated with this fact (P<0.005) were the presence of cancer and immunosuppression, which differences this subgroup from overall results. The results of the multivariate logistic regression analysis were as follows. The variables associated with in-hospital mortality were heart failure (odds ratio [OR]=5.96; 95% confidence interval [CI]: 1.93–18.47), chronic renal disease (OR=6.04; 95%CI: 1.80–20.22), necrotic infection (OR=4.33; 95%CI: 1.26–14.95), and inadequate empirical treatment (OR=44.74; 95%CI: 5.40–370.73). The variables associated with six-month mortality were chronic renal disease (OR: 3.03; 95%CI: 1.06–8.66) and a BI score of less than 20 (OR: 3.62; 95%CI: 1.17–11.21). Lastly, the variables associated with six-month readmission were male gender (OR: 2.12; 95%CI: 1.14–3.94), peripheral vascular disease (OR: 3.05; 95%CI: 1.25–7.41), and an age-adjusted CCI score of more than 3 (OR: 3.27; 95%CI: 1.40–7.63). There were no significant differences in subgroup analysis between patients with different ages, sex and type of infection when comparing in-hospital mortality, readmission or six-month mortality.
Univariate analysis. Factors associated with mortality and readmission.
| Variable | In-hospital mortality | Six-month mortality | Six-month readmission | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. (%) Died (46) | No. (%) Survivors (262) | P | No. (%) Died (21/262) | No. (%) Survivors (241/262) | P | No (%) Readmitted (69/241) | No. (%) Not readmitted (172/241) | P | |
| Male sex | 22 (47.8) | 134 (51.1) | NS | 11 (52.4) | 112 (46.5) | NS | 44 (63.8) | 88 (51.2) | 0.016 |
| Comorbid condition | |||||||||
| Vascular disease | 11 (23.9) | 25 (9.5) | 0.005 | 1 (4.8) | 22 (9.1) | NS | 14 (20.3) | 11 (6.4) | 0.001 |
| Heart failure | 26 (56.5) | 87 (33.2) | 0.003 | 9 (42.9) | 71 (29.5) | NS | 27 (39.1) | 57 (33.1) | NS |
| Chronic renal disease | 18 (39.1) | 28 (10.7) | <0.001 | 6 (28.6) | 29 (12) | 0.050 | 13 (18.8) | 25 (14.5) | NS |
| Cancer | 14 (30.4) | 33 (12.6) | 0.002 | 5 (23.8) | 27 (11.2) | NS | 14 (20.3) | 19 (11) | 0.020 |
| Dementia | 10 (21.7) | 26 (9.9) | 0.022 | 6 (28.6) | 19 (7.9) | 0.004 | 6 (8.7) | 19 (11) | NS |
| Diabetes mellitus | 18 (39.1) | 76 (29) | NS | 9 (42.9) | 59 (24.5) | NS | 22 (31.9) | 52 (30.2) | NS |
| Glucocorticoids | 5 (10.9) | 15 (5.7) | NS | 2 (9.5) | 10 (4.1) | NS | 4 (5.8) | 11 (6.4) | NS |
| Immunosuppression | 18 (39.1) | 54 (20.6) | 0.007 | 6 (28.6) | 44 (18.3) | NS | 18 (26.1) | 36 (20.9) | NS |
| Liver disease | 5 (10.9) | 32 (12.2) | NS | 2 (9.5) | 29 (12) | NS | 10 (14.5) | 22 (12.8) | NS |
| Barthel's Index<20 | 9 (19.6) | 27 (10.3) | NS | 5 (23.8) | 20 (8.3) | 0.032 | 7 (10.1) | 19 (11) | NS |
| Charlson's Index>3 | 44 (95.7) | 193 (73.9) | 0.001 | 21 (100) | 159 (66) | 0.004 | 61 (88.4) | 130 (75.6) | 0.002 |
| Origin | |||||||||
| HA infection | 23 (50) | 113 (43.1) | NS | 10 (47.6) | 96 (39.8) | NS | 38 (55) | 75 (43.6) | 0.030 |
| Nosocomial | 6 (13) | 22 (8.4) | NS | 1 (4.8) | 17 (7) | NS | 6 (8.7) | 16 (9.3) | NS |
| Form of presentation | |||||||||
| Fever | 25 (54.3) | 145 (55.3) | NS | 8 (38) | 129 (53.5) | NS | 30 (43.4) | 111 (64.5) | 0.034 |
| Sepsis | 22 (47.8) | 101 (38.5) | NS | 8 (38) | 90 (37.3) | NS | 25 (36.2) | 75 (43.6) | NS |
| Necrotic infection | 15 (32.6) | 19 (7.3) | <0.001 | 1 (4.8) | 15 (6.2) | NS | 9 (13) | 9 (5.2) | 0.022 |
| Microorganism | |||||||||
| GP infection | 12 (26.1) | 52 (19.8) | NS | 9 (42.9) | 39 (16.2) | NS | 16 (23.2) | 34 (19.8) | NS |
| GN infection | 10 (21.7) | 43 (16.4) | NS | 9 (42.9) | 30 (12.4) | NS | 21 (30.4) | 21 (12.2) | 0.037 |
| Anaerobic infection | 2 (4.3) | 9 (3.4) | NS | 0 (0) | 6 (2.5) | NS | 3 (4.3) | 6 (3.5) | NS |
| MB infection | 13 (28.3) | 33 (12.6) | NS | 7 (33.3) | 22 (9.1) | NS | 15 (21.7) | 17 (9.9) | NS |
| Treatment | |||||||||
| Surgical treatment | 4 (8.7) | 25 (9.5) | NS | 0 (0) | 23 (9.5) | NS | 12 (17.4) | 12 (7) | 0.007 |
| Inadequate treatment | 21 (45.7) | 30 (11.4) | <0.001 | 2 (9.5) | 26 (10.8) | NS | 11 (15.9) | 19 (11) | NS |
| Treatment in ICU | 10 (21.7) | 20 (7.6) | 0.004 | 3 (14.3) | 15 (6.2) | NS | 6 (8.7) | 13 (7.6) | NS |
HA: healthcare-associated. GP: gram-positive. GN: gram-negative. MB: multi-bacterial. ICU: Intensive-care Unit. NS: no significance.
Our study describes de main characteristics of a cohort of medical patients diagnosed with SSTIs. We found a higher prevalence of MR gram-negative bacteria and ESBL-producing bacterial as etiologic agent of these infections. We also found variables lined to a poorer prognosis in our patients, which could be useful to identify high risk patients and treat them more aggressively since the diagnosis.
The incidence of SSTIs (100 admissions per 500000 persons/year) in our environment is similar to that reported by other authors in Spain1 but lower than in the United States (USA).14 As reported elsewhere, the most frequent comorbidities were heart failure and diabetes mellitus.5,9
The study population's degree of dependence was low, with only 11.7% registering a BI score of less than 20. Although there were no previous data against which to compare this finding, this might well explain the low percentage of pressure ulcers observed in our series.
Local cultures were performed in 46.8% and tested positive in 66% of cases (95/144); blood cultures were performed in 70.8% and proved positive in 20.2% of cases (44/218). Other studies have described local culture performance in 33%–41% of patients (47%-85.8% positive)1,5,8 and blood culture extraction in 6.6%–47.8% of patients (18.8%–22.3% positive).1,5,8 In light of these data, we suggest that a correct collection of local cultures could increase the percentage of adequate empirical treatment and maybe the routine extraction of blood cultures could be not always necessary (e.g. patients with difficult venous access), due to the low yield of blood cultures in SSTIs.
In terms of the microbiological profile, responsible bacteria were only obtained in 95 cases. The percentage of methicillin-resistant Staphylococcus aureus (MRSA) corresponds to the prevalence of positive cultures for MRSA at our hospital (approximately 18%) but is lower than the percentage found at other health facilities around Spain (approximately 30%)3,4 and the USA (as high as 46% in some cohorts), where this microorganism is the leading cause of SSTIs.6 In contrast to reports from other countries, we observed a higher proportion of ESBL-producing Enterobacteriaceae (8.4%), mainly in healthcare-associated infections (12.2%). In our series, the presence of these bacteria was not associated with prior surgery or the presence of cirrhosis, as reported by other authors,15,16 which probably indicates the existence of a high prevalence of ESBL-producing Enterobacteriaceae skin colonisation and transmission in nursing homes in our area, rather than MRSA or other MR gram-positive bacteria.
If we focus in the empirical antibiotic treatment, attention should be drawn to the low use of active antibiotics for MRSA, such as vancomycin (2.9%) and linezolid (6.8%), and the absence of use of daptomycin and tigecycline. This finding is attributable to the low prevalence of MRSA at our hospital and to the fact that these antibiotics are recommended as empirical treatment in nosocomial infections, which were uncommon in our study.
The frequent absence of response to the initial empirical treatment in our study may be explained by the high incidence of gram-negative bacteria (55.8%), which are usually less common in SSTIs than are gram-positive bacteria.1,4,5 Other authors have likewise reported both gram-negative and multi-bacterial aetiology as factors linked to inappropriate initial antibiotic treatment.17 Hence, while empirical treatment of SSTIs is traditionally focused on gram-positive bacteria, in view of our results the need for empirical antibiotic coverage against gram-negative bacteria should perhaps be reassessed. Some factors linked to an absence of response to the initial antibiotic treatment, like previous surgery, nosocomial origin or multi-bacterial aetiology, could reflect a different microbiological profile of SSTISs in these patients. Furthermore, in the light, not only of our findings, but also of the relevance of these infections and their impact on mortality and poor prognosis,18,19 we suggest that the possibility of ESBL-producing bacteria be borne in mind when it comes to establishing an empirical treatment in health-care associated SSTIs.
In-hospital mortality was higher than that reported elsewhere (0.2%–10.9%).1,5,7,8 This finding might be related to a higher prevalence of multiple comorbidities and to the above-mentioned differences in the microbiological profile of SSTIs. Thus, our study included a 56% of patients with at least 2 chronic diseases, much higher than previous studies, with a prevalence of patients with any chronic disease near to 50%.1,5 Regarding the variables linked to in-hospital mortality, some of these factors have previously been described in surgical patients,1,8,20 to our knowledge there are no previous reports, such as ours, which describe in-hospital mortality associated with SSTIs in medical patients alone. The association between heart failure and in-hospital mortality could be due to the capital role of SSTIs as a precipitating factor of congestive heart failure episodes. As regards chronic renal disease, a lower antibiotic dosage is common in these patients, which could lead to a reduced treatment efficacy.21 In contrast, we found that in-hospital mortality was not associated with the existence of sepsis or septic shock, as in other series8 and neither with other conditions like diabetes mellitus or liver disease.1 These differences might reflect the higher complexity of our patients and the higher prevalence of cardiovascular and concomitant renal disease. The relevance of our findings therefore highlights the importance of correct identification of high-risk patients and provides tools for drawing up a more intensive treatment strategy for such patients.
Mortality across the six-month follow-up has, to our knowledge, not previously been analysed. The high mortality found in our study probably reflects the severity and repercussion of SSTIs in patients with multiple comorbidities. In this regard, the main factors associated with six-month mortality were chronic renal disease and a BI score of less than 20, which reflect greater frailty and poor prognosis. While the usefulness of BI and other indices to predict a higher mortality risk in aged and polypathological patients is well known,22,23 this study points, for the first time, to a potential role in SSTIs, by identifying high-risk patients. There are no previous data relating to the readmission risk in such infections. Our results suggest that vascular compromise and mobility limitations could lead to greater difficulty in healing and tissue regeneration, rendering it necessary to readmit these patients in order to optimise their care.
In summary, we propose that the variables associated with mortality and readmission (male gender, a BI score of less than 20, an age-adjusted CCI score of more than 3, heart failure, chronic renal disease, peripheral vascular disease and necrotic infection) could be useful for stratifying SSTI patients according to their respective risks of a poor outcome.
The main limitation of this study lies in the retrospective nature of the analysis, which could compromise the accuracy of some of the clinical data included. In addition, the absence of an established protocol for SSTI management at our hospital might be an important bias, due to the lack of uniformity in the diagnosis and treatment of such patients. Finally, the inclusion of the absence of response to the initial antibiotic treatment due both to clinical and microbiological criteria could also be a significant bias.
ConclusionsThe relevance of SSTIs as a health problem in our area is high, as is the impact of these diseases on mortality, principally among patients with multiple comorbidities. There are clinical variables, such as heart failure, chronic renal disease, peripheral vascular disease and necrotic infection, which could help identify high-risk patients. The main factor associated with higher mortality is inadequate initial empirical treatment, something that is very common; in the light of our findings, physicians should consider gram-negative bacteria and even ESBL-producing bacteria when designing the initial empirical treatment for SSTIs, particularly in health-care associated cases. In this regard, correct extraction of local cultures could both help and guide antibiotic treatment strategy.
FundingNo funding was required to develop this work.
Conflict of interestAll authors declare that they have no conflict of interest.