The epidemiology of S. aureus depends on conditions in specific populations. Few studies of S. aureus colonization in the older population have been performed in Spain. The aim of this study was to determine the prevalence of methicillin-resistant S. aureus (MRSA) colonization and its molecular epidemiological characteristics in an institutionalized population in community residential care homes in Cadiz, Spain.
MethodsA cross-sectional epidemiological study was conducted in three residential care homes for older people. Axilla and nostril samples were tested. Identification of S. aureus and antimicrobial susceptibility testing were by MALDI-TOF and MicroScan panels. MRSA strains were subjected to SCCmec typing, multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE). The presence of Panton–Valentine leukocidin (PVL) genes was determined by PCR in all S. aureus strains.
ResultsA total of 293 residents were included. Fifty-one residents (17.4%) were colonized with methicillin-sensitive S. aureus (MSSA) and 11 (3.8%) with MRSA. Resistance to at least two aminoglycosides was observed in 25.4% of MSSA and 90.9% and of MRSA isolates, and resistance to levofloxacin in 80.3% of MSSA and 100% of MRSA isolates. SCCmecIV was detected in all isolates and all except one (ST-125) were ST-8. None of the S. aureus isolates were positive for PVL.
ConclusionsA low rate of S. aureus carriage was detected and the prevalence of MRSA was very low. ST8-MRSA-IVc was the dominant clone, and only one strain belonged to ST125-MRSA-IVc. We found MRSA transmission within the residential care homes and a very high rate of quinolone resistance in MSSA and MRSA.
La epidemiología de S. aureus depende de las condiciones particulares de cada población. En España se han realizado pocos estudios sobre la colonización por S. aureus en la población geriátrica. El objetivo de este estudio es determinar la prevalencia de colonización por S. aureus resistente a meticilina (SARM) y sus características epidemiológicas moleculares en población institucionalizada en residencias de ancianos en Cádiz, España.
MétodosSe realizó un estudio epidemiológico transversal en 3 residencias de ancianos. Se estudiaron muestras de las fosas nasales y axilas. La identificación y las pruebas de sensibilidad se realizaron utilizando MALDI-TOF y paneles MicroScan®. En los aislados de SARM se determinó el tipo de SCCmec y se tiparon mediante Multilocus Sequence Typing (MLST) y Pulsed-field Gel Electrophoresis (PFGE). La presencia de genes de la leucocidina de Panton-Valentine (LPV) se determinó mediante PCR en todas las cepas de S. aureus.
ResultadosSe incluyeron un total de 293 residentes. Cincuenta y un residentes (17,4%) estaban colonizados por S. aureus sensible a la meticilina (SASM) y 11 (3,8%) por SARM. Se observó resistencia frente al menos 2 aminoglucósidos en el 25,4 y 90,9% y resistencia a levofloxacino en el 80,3 y 100% de los aislamientos de SASM y SARM, respectivamente. Se detectó SCCmecIV en todos los aislados, y todos, excepto uno (ST-125) correspondían al ST-8. Ninguno de los aislados de S. aureus fue positivo para LPV.
ConclusionesSe detectó una baja tasa de portadores de S. aureus, siendo el porcentaje de SARM muy bajo. ST8-MRSA-IVc fue el clon predominante, y solo una cepa pertenecía a ST125-MRSA-IVc. Se objetivó transmisión de SARM intracentro. Se observó una tasa muy alta de resistencia a quinolonas en SASM y SARM.
Staphylococcus aureus is a highly versatile pathogen of great importance in human medicine. It is capable of causing a wide variety of diseases, ranging from food poisoning and skin infections to life-threatening conditions such as bacteremia, pneumonia, osteomyelitis and endocarditis. Besides its pathogenic advantages and ability to develop resistance mechanisms, S. aureus is able to colonize humans, primarily the nose.1 Colonization is an important factor in the pathogenesis and epidemiology of infections due to methicillin-susceptible S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA). MRSA colonization is known to increase the risk of subsequent infection in hospital patients and nursing home residents.2
The population dynamics of MRSA is undergoing significant change as a result of demographic variations due to the development of more sophisticated and complex healthcare systems (long-term residential care facilities, for example).3 The epidemiology of S. aureus depends on the characteristics of specific populations. In Spain, there are few studies of S. aureus colonization in the elderly population or describing the molecular characteristics of colonizing strains. We conducted a cross-sectional study in order to determine the prevalence and molecular epidemiology of MRSA colonization in an institutionalized population in community nursing homes in Cadiz, Spain.
Patients, materials and methodsA cross-sectional epidemiological study was conducted between September 2016 and May 2017, involving three nursing homes in Cadiz, Spain. A total of 359 eligible residents were asked to provide written informed consent. The distribution of patients was as follows: Center 1 (n=160 residents), Center 2 (n=120 residents) and Center 3 (n=79 residents), with nurse/patient ratios of 0.05, 0.12 and 0.08, respectively. All patients were accommodated in double-rooms. The study was conducted in accordance with the Declaration of Helsinki4 and approval was obtained from local ethics committees. If a resident was unable to provide informed consent because of documented cognitive difficulties, written informed consent was obtained from their durable power of attorney. Demographic data (age, sex, comorbidities (Charlson scale), functional status (Barthel scale), antibiotic therapy and hospitalization in the last three months) were obtained by chart review.
Samples were obtained on a single day (point prevalence screening) from the anterior nares and axillary areas, which were then streaked onto mannitol salt agar (MSA) and incubated at 35°C for 48h. Bright yellow colonies of S. aureus grown on mannitol agar were identified by MALDI-TOF MS (MALDI-TOF Biotyper 3.1; Microflex, Bruker). Antimicrobial susceptibility testing was performed using the commercialized microdilution PM 33 MicroScan panels (Beckman Coulter, Spain). A cefoxitin disk diffusion method was also used, following EUCAST recommendations (www.eucast.org).
SCCmec typing was carried out with multiplex PCR, using previously described primers and conditions.5SCCmec amplification products were analyzed by agarose gel electrophoresis, and amplicon size was determined by comparison with a 100bp Lambda DNA ladder (Invitrogen, Carlsbad, CA, USA). Types were then assigned according to the size of the different fragments obtained. Further PCR typing was performed to detect SCCmec IV subtypes IVa, IVb, IVc, IVd, IVg and IVh.6
Multilocus sequence typing, amplifying fragments of arcC, aroE, glpF, gmk, pta, tpi, and yqiL housekeeping loci, was performed on all MRSA strains. All amplicons were sequenced, and allelic profiles and ST types were assigned using the MLST database (http://www.mlst.net).7
PFGE analysis of SmaI-digested DNA was used to determine the degree of genetic relatedness between isolates.8 A dendrogram was constructed with Fingerprinting 3.0 software (Bio-Rad, Madrid, Spain) using the Dice coefficient and position tolerance settings: 1% optimization and 0.85% band position tolerance. Isolates were assigned to different pulsotypes if the similarity coefficient was <80%. Different subtypes were considered for similarity coefficients fluctuating in the 80–95% interval.9
The presence of Panton–Valentine leukocidin (PVL) genes (lukS-PV and lukF-PV) was determined by PCR in all S. aureus strains, using the method described by Lina et al.10 A PVL-positive strain was used as a positive amplification control.
ResultsA total of 293 residents were included; 20 patients refused to give their consent and 46 were unable to do so and their durable power of attorney agents could not be contacted. Median age in years was 83.9 (range: 50.7–99.6) and 61.1% were women. Thirty-eight per cent of residents had a Charlson comorbidity score ≥3 and 46.1% scored less than 40 points on the Barthel scale. Thirty-seven per cent of residents had received at least one course of antibiotics in the previous three months.
Overall, 51 (17.4%) of 293 residents were colonized with MSSA and eleven (3.8%) with MRSA. No cases of S. aureus infection were detected during the study. With respect to individual nursing homes, the percentages of MSSA and MRSA were as follows: Center 1: 16%, 6%; Center 2: 14%, 1%; Center 3: 24%, 3%. No significant differences in colonization rate by center were found.
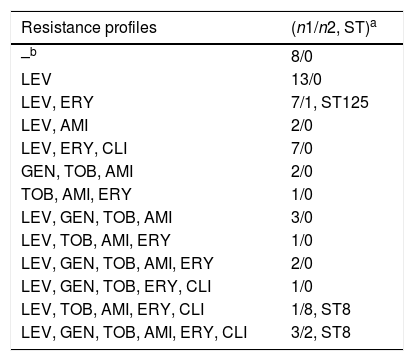
Ten MSSA isolates (19.6%) showed resistance to amikacin, tobramycin and gentamicin, and three (5.8%) to tobramycin and amikacin. Forty-one isolates (80.3%) were resistant to levofloxacin. With respect to macrolides, the cMLS phenotype was detected in 23.6% of MSSA isolates, and the M phenotype in 21.5% (Table 1).
Antimicrobial resistance profiles of all isolates included in the study.
| Resistance profiles | (n1/n2, ST)a |
|---|---|
| –b | 8/0 |
| LEV | 13/0 |
| LEV, ERY | 7/1, ST125 |
| LEV, AMI | 2/0 |
| LEV, ERY, CLI | 7/0 |
| GEN, TOB, AMI | 2/0 |
| TOB, AMI, ERY | 1/0 |
| LEV, GEN, TOB, AMI | 3/0 |
| LEV, TOB, AMI, ERY | 1/0 |
| LEV, GEN, TOB, AMI, ERY | 2/0 |
| LEV, GEN, TOB, ERY, CLI | 1/0 |
| LEV, TOB, AMI, ERY, CLI | 1/8, ST8 |
| LEV, GEN, TOB, AMI, ERY, CLI | 3/2, ST8 |
SCCmecIVc was detected in all MRSA isolates. ST8 was the main genotype and was detected in all isolates except one (ST125). MRSA antimicrobial susceptibility profiles showed that 2 isolates (18.2%) were resistant to amikacin, tobramycin and gentamicin, and 8 isolates (72.7%) to tobramycin and amikacin. Only one isolate (ST125-MRSA-IVc) was susceptible to all aminoglycosides. All isolates were resistant to levofloxacin. Moreover, ten ST8-MRSA-IVc isolates (90.1%) were resistant to erythromycin and clindamycin (cMLS phenotype) and the M phenotype was detected only in 1 (ST125-MRSA-IVc) (Table 1).
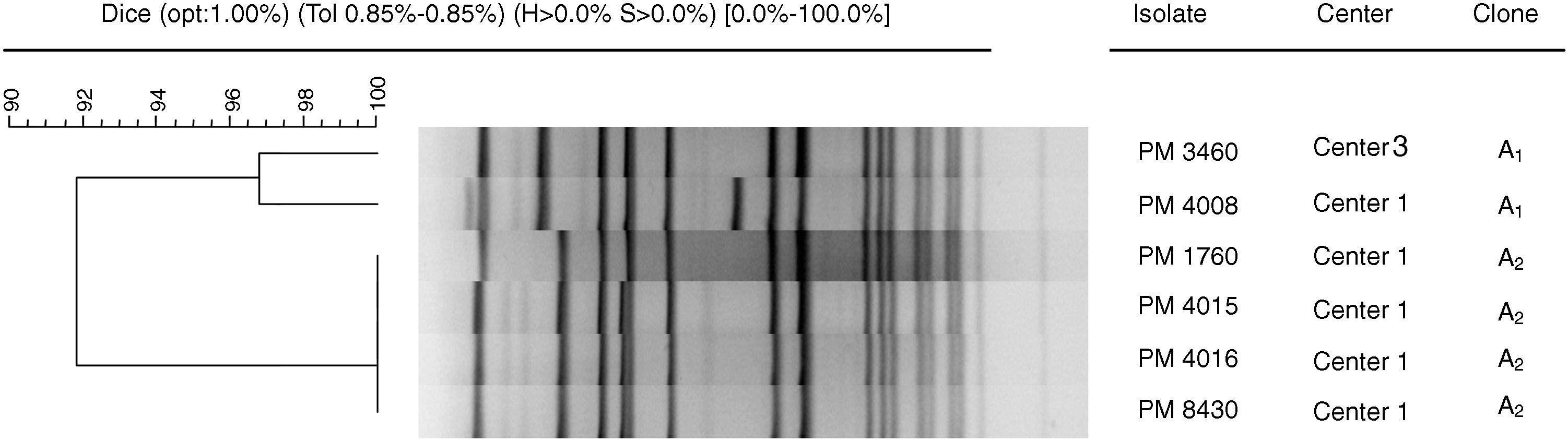
Six isolates, corresponding to ST8-MRSA-IVc (five from Center 1 and one from Center 3) were selected in order to determine the degree of genetic relatedness by PFGE and to assess inter- and intra-nursing home transmission of MRSA. All isolates belonged to the same clone, although two subtypes (91% similarity) were distinguished: A1 (with two pulsotypes, 97% similarity) and A2 (100% similarity) (Fig. 1).
Dendrogram of SmaI-PFGE patterns illustrating genetic relationships between six S. aureus isolates: five from Center 1 and one from Center 3. The dendrogram was created with Fingerprinting 3.0 software (Bio-Rad, Madrid, Spain), using the Dice coefficient with position tolerance settings: 1% optimization and 0.85% position tolerance.
None of the 62 S. aureus isolates were positive for the Panton–Valentine leukocidin.
DiscussionThe aim of this study was to determine the prevalence of S. aureus carriage among residents of three community nursing homes with different characteristics in terms of size, number of residents and health personnel, and to characterize the MRSA isolates.
MRSA colonization is known to increase the risk of subsequent infection in hospitalized patients and nursing home residents and can play an important role in the regional spread of MRSA.11
In our study, 17.4% of residents were colonized with MSSA and 3.8% with MRSA. These percentages are lower than those reported in community nursing home settings in the USA (21.2% MSSA and 40.7% MRSA).3 In Belgium, Denis et al.12 conducted a cross-sectional study on the prevalence of MRSA carriage among residents in nursing homes and found that 19.9% of residents were MRSA carriers. In Hamburg, however, the number of cases of MRSA colonization among residents of geriatric nursing homes was rather low, 5.5%.13 Similar results have been found in other European countries, with a prevalence of 0% and 0.3% reported in Sweden14 and the Netherlands,15 respectively. Results of a cross-sectional study among subjects living in long-term-care facilities in southern Spain during 2009–2010 showed that 79 (10.6%) and 67 (9%) were colonized by MRSA and MSSA, respectively.16 In our setting, this low prevalence could be explained by the implementation of effective hand-washing guidelines.
In our study, 80.3% of MSSA isolates exhibited quinolone resistance, which is a much higher rate than that described by Lozano et al. in strains isolated from healthy humans in Spain (3.8%).17 There are two possible explanations for this situation. First, antibiotics are among the most commonly prescribed drugs in long-term care facilities, accounting for nearly 40% of all prescribed drugs and there is a 70% likelihood of residents receiving at least one course of antibiotics per year. A high percentage of antibiotic treatments are considered inappropriate, which contributes to increased antimicrobial resistance in the elderly population.18 Second, the pattern of antibiotic resistance seems to be correlated with the age of patients. In their recent study of 511 cases of MRSA infection, García et al. concluded that antibiotics that target DNA synthesis (namely fluoroquinolones) result in progressively higher numbers of resistant isolates in the older population.19
All the MRSA isolates in our study were resistant to levofloxacin and almost 91% were resistant to two or three aminoglycosides. These results are similar to those reported by Rodríguez-Baño et al.20 for healthcare-associated MRSA strains in Spain.
SCCmecIVc was detected in all MRSA isolates in our study and ST8 was detected in all isolates except one (ST125). SCCmec type IV is not a good marker for community isolates in Spain, because it is the most frequent type in healthcare-associated and nosocomial isolates.20,21
In a recent study performed by Moreno-Flores et al., the major clonal type detected was ST8-MRSA-IVc (32.6%) in isolates with the oxacillin-resistant only phenotype (32.6%) and ST8 levofloxacin-resistant strains were not identified.22 Differences between this study and ours could be explained by the different populations included. Another interesting finding is that no PVL-positive (MRSA or MSSA) isolates were found in our study. The initial spread of a clone with genetic traits related to those of the USA300 clone (ST8-MRSA-IV-PVL+) has been reported in Spain and seems to be associated with community-acquired infections,23 although at least one case of healthcare-associated infection due to this clone has also been detected.20 Our study has also demonstrated intra-nursing home transmission of ST8-MRSA-IVc.
In the study performed by Vindel et al. in 145 Spanish hospitals, ST125-MRSA-IV was the most prevalent clone in Spain.24 In our study, however, only one isolate belonged to this sequence type. This lineage is associated with aminoglycoside resistance and, to a lesser extent, macrolide resistance and has been reported as causing bacteremia in a Spanish geriatric population.25 Moreno-Flores et al.22 found that strains belonging to this sequence type were levofloxacin-resistant. Although the only ST125-MRSA-IVc isolate in our study showed susceptibility to all aminoglycosides tested, it was resistant to macrolides and levofloxacin.
The limitations of our study are related to the small number of positive results, which makes difficult an adequate statistical analysis. However, our results could be extrapolated to centers with similar characteristics with effective hand-washing implementation. More studies should be conducted in the future to gain knowledge concerning the prevalence and the genetic lineages of S. aureus circulating among nursing homes of different characteristics.
In summary, there was a low rate of S. aureus carriage and a very low prevalence of MRSA detected in the elderly population in our area. ST8 was the predominant clone and only one strain belonged to ST125, although the latter sequence type is considered to be one of the predominant Spanish clones. We observed an evidence of MRSA transmission within nursing homes and a very high rate of quinolone resistance was also observed among MSSA and MRSA isolates, probably associated with the characteristics of the population included in the study.
Conflict of interestThe authors declare no conflict of interest.