Reactive oxygen species (ROS) overgeneration is involved in the pathogenesis of hepatitis C. The aim of this study was to evaluate the antioxidant status in the blood of HCV infected patients treated or not with standard therapy before and after supplementation of vitamins E, C and zinc. Biomarkers of oxidative stress were evaluated in the blood of three groups of patients: group 1 – controls; group 2 – HCV patients without treatment examined before and after a daily antioxidant supplementation (vitamin E 800mg, C 500mg and zinc 40mg) for 6 months; and group 3 – HCV patients treated with pegylated interferon combined with ribavirin, also examined before and after the same antioxidant supplementation. Before antiviral treatment HCV patients showed enhanced superoxide dismutase, catalase and glutathione peroxidase activities and decreased glutathione reductase activity, while lipoperoxidation was increased and reduced glutathione showed decreased levels compared to controls. Treatment with standard therapy enhanced the activities of catalase and glutathione S-transferase, increased contents of protein carbonyl and promoted further reduced glutathione depletion. After antioxidant supplementation, decreased catalase and glutathione S-transferase activities, decreased lipoperoxidation in group 2, and increased reduced glutathione contents in both supplemented groups were detected. Before antioxidant supplementation, alanine aminotransferase and gamma glutamyl transferase contents showed significant increases in group 2. Conclusion: Untreated HCV patients and also those treated with the standard therapy are coping with a systemic oxidative stress. The antioxidant supplementation conferred an antioxidant protection to both supplemented groups attenuating oxidation processes related to the disease.
La generación excesiva de especies reactivas de oxígeno está implicada en la patogénesis de la hepatitis C. El objetivo de este estudio fue evaluar el estado antioxidante de la sangre en pacientes infectados por VHC tratados o sin tratamiento con la terapia estándar, antes y después de la complementación con vitaminas E, C y Zinc. Se evaluaron los biomarcadores de estrés oxidativo en sangre de tres grupos de pacientes: grupo 1 - controles, grupo 2 - pacientes con VHC sin tratamiento examinados antes y después de una complementación diaria de antioxidantes (800mg de vitamina E, 500mg de vit. C, y 40mg de zinc) durante 6 meses y grupo 3 - pacientes con VHC tratados con interferón pegilado combinado con ribavirina, también examinados antes y después de la misma complementación diaria con antioxidantes. Antes del tratamiento antiviral los pacientes con VHC mostraban una mayor actividad del superóxido dismutasa, la catalasa y el glutatión peroxidasa y una actividad reducida de la glutatión reductasa, mientras que la lipoperoxidación estaba incrementada y el glutatión reducido presentaba niveles bajos comparados con los del grupo control. El tratamiento con la terapia estándar potenciaba la actividad de la catalasa y del glutatión S-transferasa, aumentaba el contenido de proteínas carbonilo y fomentaba un mayor uso del glutatión reducido. Después de la complementación con antioxidantes, en el grupo 2 disminuyó la actividad de la catalasa y del glutatión S-transferasa, disminuyó la lipoperoxidación, y aumentó el contenido de glutatión reducido en los dos grupos con complementos. Antes de la complementación con antioxidantes, el contenido de alanina aminotransferasa y gamma glutamil transferasa mostraban incrementos significativos en el grupo 2. Conclusión: Los pacientes con VHC sin tratar y aquellos tratados con la terapia estándar hacen frente a un estrés oxidativo sistémico. Los complementos con antioxidantes confieren una protección antioxidante a los dos grupos tratados y atenúan los procesos de oxidación relacionados con la enfermedad.
Virus hepatitis C (HCV) has a universal distribution and its magnitude varies according to regional aspects from different countries. The WHO1 estimated that around 170 million people are infected by HCV, about 3% of the world population. HCV is the leading cause of acute hepatitis and chronic liver disease, which may lead to cirrhosis and hepatocellular carcinoma.2
The combined therapy with interferon with or without pegylation associated with ribavirin has shown greater sustained virological response than monotherapy with interferon-α. However, this response still represents around 60% of cases of hepatitis C with genotype 1.2 The mechanisms by which HCV causes cellular damage are not yet well understood, albeit immune liver damage, direct cytotoxic damage mediated by different viral products and also oxidative stress have been implicated in the pathogenesis of chronic hepatitis C.2 Studies support that ROS and oxidative stress are involved in the pathogenesis of hepatitis C,3,4 despite that increased ROS levels in HCV infected patients might be beneficial by suppressing HCV replication.5 However, contradictory results were obtained on the combined efficacy of antioxidants/interferon/ribavirin regarding the treatment of naive patients with chronic hepatitis C.2–4 Essential micronutrients are involved in protein synthesis, immunology and antioxidant defenses.6,7 Zinc is an important cofactor to several enzymes and also participates in the structure of metalothionein.8
The objective of the present study was to evaluate the antioxidant status in the blood of patients infected HCV treated with pegylated interferon combined with ribavirin before and after supplementation of vitamins E, C and mineral zinc during six months.
Patients and methodsExperimental designTwelve healthy subjects (7 males and 5 females; average age of 39.9±11.8 years) were recruited from the Blood Bank of the University Hospital from the Federal University of Santa Catarina, Brazil, to constitute the Control group, or group 1. All subjects were negative for HCV, HBV, HIV, HBsAg, anti-HBc total, anti-HCV and normal serum transaminases. These patients followed criteria for inclusion such as age between 20 and 60 years with potential to become pregnant and without the presence of illnesses associated with systemic diseases, no chronic alcoholism, without HIV coinfection, and were not participating in other studies. Patients with one of the following laboratory abnormalities were also excluded: leucocytes <2000/mL, neutrophils <1000/mL, platelets <50,000/mL, serum creatinine >1.5 times upper limit of normal, elevated thyroid stimulating hormone, α-fetoprotein above normal limits, and/or focal lesion on ultrasound performed within 1 month of study entry.
Twelve subjects infected with HCV non-treated for the disease were recruited (7 males and 5 females with an average age of 45.0±9.8 years; HCV group, or group 2) from the local Policlinic 2, however, only nine patients participated in the final analysis, whereas one patient withdrew and two did not participate for other reasons.
The third group of patients composed of 14 individuals with hepatitis C treated with pegylated interferon combined with ribavirin, were recruited (11 males and 3 females, average age of 47.0±7.6 years; treated HCV patients or group 3) from the local hospital Nereu Ramos. Only 11 participated in the final analysis because three patients withdrew themselves.
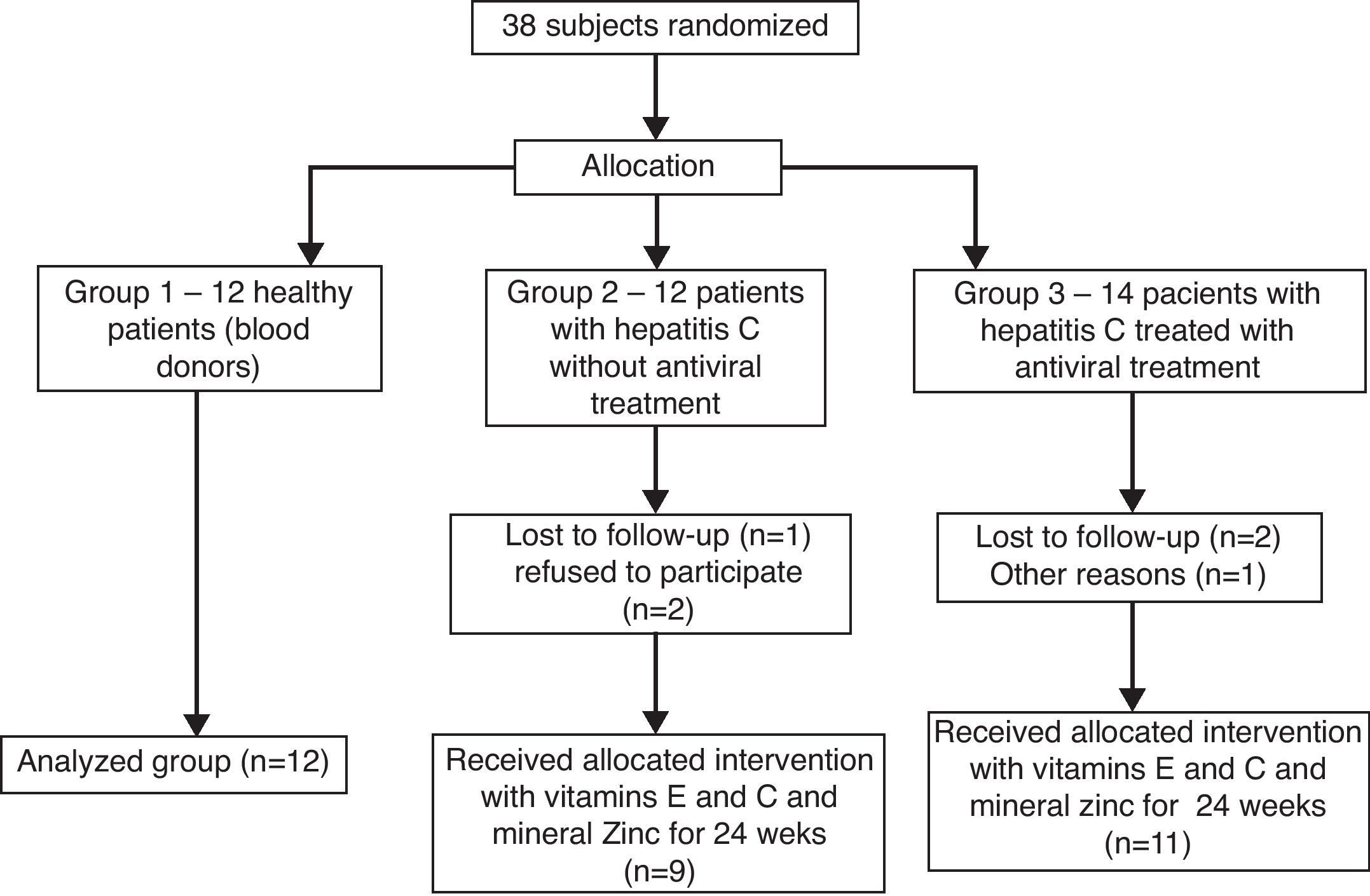
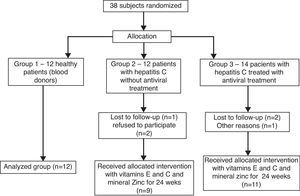
The patients infected with HCV were allocated according to the presence or not of the antiviral therapy with interferon and ribavirin (groups 3 and 2, respectively), all of them receiving the antioxidant supplementation for 6 months, while controls (group 1) did not receive both treatments. Therefore, 38 patients were recruited for the study, of which 12 healthy subjects were included in group 1, 12 patients with hepatitis C without antiviral treatment were allocated in group 2, and 14 patients with hepatitis C treated with interferon associated with ribavirin were allocated in group 3, thus 38 subjects started the treatment for 24 weeks (Fig. 1).
Diagnosis for hepatitis C was based on the risk of elevated antibodies to HCV positive and presence of HCV-RNA in serum, and histological evidence of chronic hepatitis. Patients with hepatitis C were selected according to the Clinical Protocol and Guidelines for Therapeutic Hepatitis C Viral.9 Clinical data were obtained consecutively, through the enrollment of patients in the clinic and through the data contained in medical records. The initial assessment of patients with hepatitis C treated with pegylated interferon combined with ribavirin occurred after 12 weeks of treatment.
A 24-h period of a dietary recall and food frequency questionnaire was carried out to calculate the approximate nutritional intake and to get information on the nutritional antioxidant intake of all subjects, keeping however their nutritional habits.
To carry out this trial, this protocol was sent and approved by the Research Ethics Committee of UFSC (Protocol 120/07-CEP), and is registered in the Clinical Trials US National Institutes of Health (Protocol NCT00983164). The experimental protocol attended the rules of the resolution 196/1996 of the National Board of Health on Clinical Research, as well as ethical, scientific and technical consonant with the standards of international acceptance for clinical trials (standards of “Good Clinical Practice”). Each patient completed a Free and Informed Consent before blood collection.
Evaluation of food consumptionNutritional anamnesis was performed using the 24-h food recall and food frequency questionnaire to calculate food intake, according to the program NUTWIN from the University of São Paulo, 1.5.2.51-2005 version, using as a database of the chemical composition TACO food to the table (UNICAMP, version 2), with the aim of preserving the nutritional diet of patients.
Interferon and ribavirin treatmentPatients with hepatitis C were orally administered for pegylated alpha-interferon 2a and 2b, 1.5μg/kg and 180μg, respectively, once a week, combined with ribavirin, 1000–1250mg once a day (1000mg per day for patients under 75kg and 1250mg per day for patients with 75kg or more), according to the Clinical Protocol and Guidelines for Therapeutic Hepatitis C Viral.9
Supplementation of vitamins E, C and zinc mineralFor the supplementation of vitamins E and C and the mineral zinc in groups 2 and 3, reference quantities suggested by Nathens and collaborators10 were used, which are based in the Upper Intake Level (UL). Vitamin E in the form of acetate dl-α-tocopherol (Galena Chemical and Pharmaceutical Ltda®, Florianópolis, Brazil) 0.8UL (≅54×RDA) dose of 800mg/day, and coated pills of vitamin C (Pharma Nostra®, Florianópolis, Brazil), 0.5UL (≅5.5×RDA), dose of 500mg/day, were used. For mineral zinc, 1UL (≅3.6×RDA) in the form of zinc chelate (Pharma Nostra®), a dose of 40mg/day was used. The extra end was composed of 4 daily capsules during meals for a period of 24 weeks.
Sample preparationA volume of 15ml of blood was collected intravenously from individuals fasted for at least 8h, and stored in liquid nitrogen. Immediately after collecting blood samples into a tube containing heparin (or without heparin to obtain serum), a whole blood fraction was immediately separated and precipitated with trichloroacetic acid 12% (1:4, v:v) in cold vials and stored in liquid nitrogen until GSH analysis. Separation of red blood cells and plasma was performed by a rapid centrifugation (3000×g for 3min at 5°C) and the corresponding aliquots were immediately stored in liquid nitrogen until analysis. To obtain the hemolysates, red cells were washed two times with saline solution, centrifuged (5000×g for 3min at 5°C) and successive freeze and thaw procedures ensured complete cell lysis, provided the hemolysate supernatant for enzymatic evaluations. TBARS contents were examined in plasma and protein carbonyl contents were examined in serum.
Determination of biochemical markers of hepatic injuryThe concentrations of AST, ALT and GGT11–13 were measured in Siemens® kits through a kinetic-colorimetric method using a EØ: BT 3000 plus Wiener Lab 1.7.6.0/RT version equipment.
Antioxidant enzyme assaysThe activity of SOD was measured at 480nm according to Misra and Fridovich,14 modified by Boveris and Cadenas15 through the oxidation of epinephrine. The activity of CAT was analyzed at 240nm according to Aebi,16 which is based on the speed of degradation of hydrogen peroxide in a freshly prepared solution. GPx determination was measured at 340nm by the method of Flohé and Gunzler.17 For GR measurements the method of Calberg and Mannervik18 was used, which checks at 340nm the rate of oxidation of NADPH due to the formation of reduced glutathione (GSH) from GSSG by the intervention of GR present in the assay. The activity of GST was measured at 340nm, using CDNB as substrate and a 0.15M GSH solution, according to Habig and collaborators.19
Reduced glutathione assay (non-protein thiols)Whole blood levels of GSH were determined using DTNB (2-dithionitrobenzoic acid) by the method of Beutler and collaborators.20
Oxidative stress biomarkersEndogenous lipid peroxidation was assayed by the detection of substances that react with thiobarbituric acid (TBARS), particularly malondialdehyde (MDA), producing a pink Schiff base detected at 535nm,21 using ¿=1.56×105M−1cm−1.
The oxidative damage to proteins was assessed by the method proposed by Reznick and Packer22 using DPNH (2,4-dinitrophenyl-hydrazine) at the absorbance range of 360–370nm. The final protein values were expressed using ¿=22mM−1cm−1.
Vitamin E contentQuantitative determination of vitamin E was performed by high pressure liquid chromatography (HPLC), using UV detection at 292nm according to the method of Nicoletti and collaborators.23
Zinc and iron contentsThe contents of Zn and Fe were determined by the PIXE technique (Particle Induced X-ray Emission), as described by Johansson and collaborators,24 at the Ion Implantation Laboratory of the Institute of Physics, Universidade Federal do Rio Grande do Sul, Brazil.
Statistical analysesData were expressed as means±SEM. Unpaired Student t test was used to compare the different groups, two by two. The Student t test was also used for the comparison between groups 2 and 3, before and after antioxidant supplementation, followed by the Duncan's multiple range test where appropriate. Minimal values of p<0.05 were considered statistically significant.
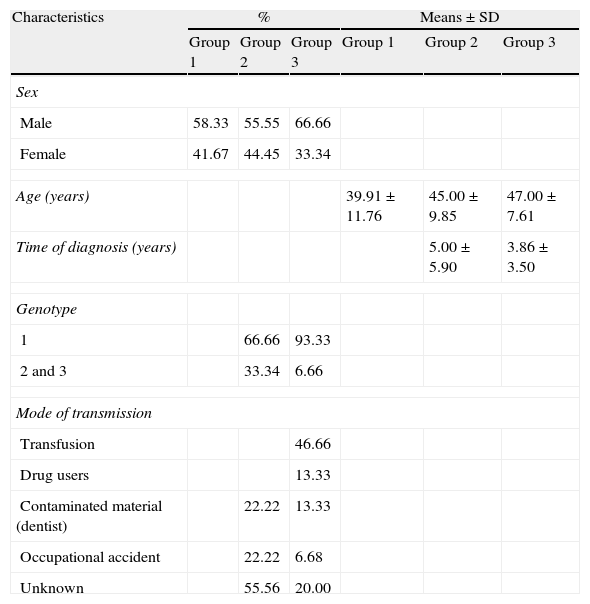
ResultsThe clinical characteristics of the three groups were homogeneous with respect to gender and age (Table 1). Regarding the time of diagnosis group 3 revealed a period lower than the group 2. The genotype predominance was type 1 and considering the mode of HCV transmission, there was a prevalence of transfusion in group 3, whereas in group 2 an unknown form prevailed (Table 1).
Clinical characteristics of groups 1, 2 and 3.
| Characteristics | % | Means±SD | ||||
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |
| Sex | ||||||
| Male | 58.33 | 55.55 | 66.66 | |||
| Female | 41.67 | 44.45 | 33.34 | |||
| Age (years) | 39.91±11.76 | 45.00±9.85 | 47.00±7.61 | |||
| Time of diagnosis (years) | 5.00±5.90 | 3.86±3.50 | ||||
| Genotype | ||||||
| 1 | 66.66 | 93.33 | ||||
| 2 and 3 | 33.34 | 6.66 | ||||
| Mode of transmission | ||||||
| Transfusion | 46.66 | |||||
| Drug users | 13.33 | |||||
| Contaminated material (dentist) | 22.22 | 13.33 | ||||
| Occupational accident | 22.22 | 6.68 | ||||
| Unknown | 55.56 | 20.00 | ||||
Note. Group 1: healthy patients; group 2: untreated patients with hepatitis C; group 3: patients with hepatitis C treated with interferon associated with ribavirin.
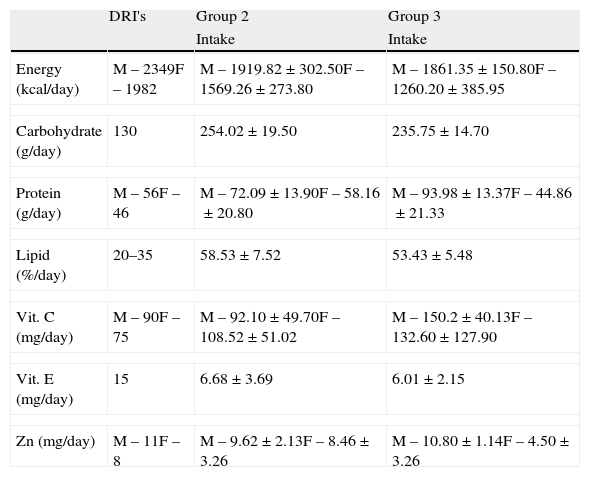
Macronutrients such as carbohydrates, lipids and proteins were above the recommendations of the RDA-DRI's,25 while the dietary intake of vitamin E presented low levels (55.5% in men from group 2 and 59.9% in women from group 3), similar to the consumption of Zn (Table 2).
Characteristics of food intake of groups 1, 2 and 3.
| DRI's | Group 2 | Group 3 | |
| Intake | Intake | ||
| Energy (kcal/day) | M – 2349F – 1982 | M – 1919.82±302.50F – 1569.26±273.80 | M – 1861.35±150.80F – 1260.20±385.95 |
| Carbohydrate (g/day) | 130 | 254.02±19.50 | 235.75±14.70 |
| Protein (g/day) | M – 56F – 46 | M – 72.09±13.90F – 58.16±20.80 | M – 93.98±13.37F – 44.86±21.33 |
| Lipid (%/day) | 20–35 | 58.53±7.52 | 53.43±5.48 |
| Vit. C (mg/day) | M – 90F – 75 | M – 92.10±49.70F – 108.52±51.02 | M – 150.2±40.13F – 132.60±127.90 |
| Vit. E (mg/day) | 15 | 6.68±3.69 | 6.01±2.15 |
| Zn (mg/day) | M – 11F – 8 | M – 9.62±2.13F – 8.46±3.26 | M – 10.80±1.14F – 4.50±3.26 |
Note: Group 1: healthy patients; group 2: untreated patients with hepatitis C; group 3: patients with hepatitis C treated with interferon associated with ribavirin.
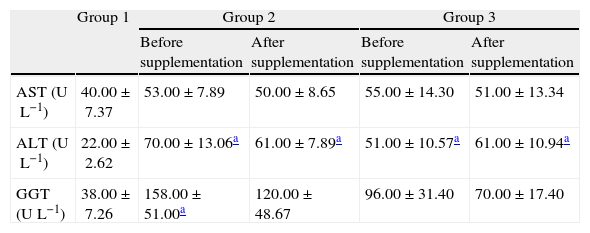
AST, ALT and GGT levels did not change among the groups with and without antiviral treatment while in the control group values of hepatic damage index were lower than those detected in groups 2 and 3, while after the antioxidant supplementation, no statistical differences in these markers were observed (Table 3).
Biochemical markers of hepatic injury measured in plasma before and after antioxidant supplementation.
| Group 1 | Group 2 | Group 3 | |||
| Before supplementation | After supplementation | Before supplementation | After supplementation | ||
| AST (UL−1) | 40.00±7.37 | 53.00±7.89 | 50.00±8.65 | 55.00±14.30 | 51.00±13.34 |
| ALT (UL−1) | 22.00±2.62 | 70.00±13.06a | 61.00±7.89a | 51.00±10.57a | 61.00±10.94a |
| GGT (UL−1) | 38.00±7.26 | 158.00±51.00a | 120.00±48.67 | 96.00±31.40 | 70.00±17.40 |
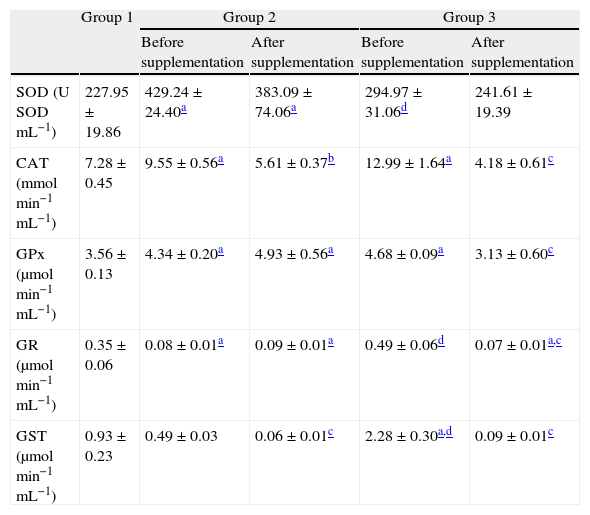
Before supplementation, SOD activity showed a significant increased activity in untreated HCV patients compared to controls and also to HCV patients treated with pegylated interferon combined with ribavirin (p<0.01; Table 4). After 6 months of antioxidant supplementation, no significant differences were detected in both supplemented groups (Table 4). Before supplementation CAT activity also showed a significant increased activity in patients from groups 2 and 3 compared to controls (p<0.01). After the antioxidant supplementation there was a significant decrease in CAT activity in patients from both supplemented groups (p<0.01; Table 4). Before the antioxidant supplementation GPx activity showed a profile similar to that of CAT, while after supplementation a significant decrease in patients from group 3 was observed. Before antioxidant supplementation patients from group 3 showed increased GR activities, which were lowered after the supplementation (p<0.01; Table 4). Before the antioxidant intervention GST activity showed a significant increase in patients from group 3 compared to those from groups 1 and 2 (p<0.01), and after the intervention showed a markedly decrease in groups 2 and 3 (p<0.01).
Antioxidant enzymatic activities in hemolysates before and after antioxidant supplementation.
| Group 1 | Group 2 | Group 3 | |||
| Before supplementation | After supplementation | Before supplementation | After supplementation | ||
| SOD (U SODmL−1) | 227.95±19.86 | 429.24±24.40a | 383.09±74.06a | 294.97±31.06d | 241.61±19.39 |
| CAT (mmolmin−1mL−1) | 7.28±0.45 | 9.55±0.56a | 5.61±0.37b | 12.99±1.64a | 4.18±0.61c |
| GPx (μmolmin−1mL−1) | 3.56±0.13 | 4.34±0.20a | 4.93±0.56a | 4.68±0.09a | 3.13±0.60c |
| GR (μmolmin−1mL−1) | 0.35±0.06 | 0.08±0.01a | 0.09±0.01a | 0.49±0.06d | 0.07±0.01a,c |
| GST (μmolmin−1mL−1) | 0.93±0.23 | 0.49±0.03 | 0.06±0.01c | 2.28±0.30a,d | 0.09±0.01c |
All values are mean±SEM; n=12 (group 1), n=9 (group 2) and n=11 (group 3).
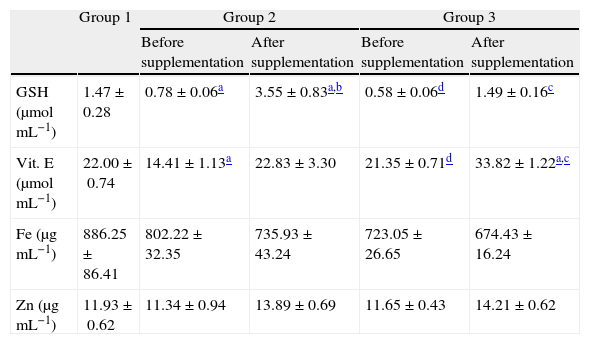
Whole blood concentrations of GSH showed depletions when comparing patients from groups 2 and 3 with controls (Table 5), values that were recovered after 6 months of antioxidant supplementation (p<0.01).
Contents of vitamin E in plasma and GSH and mineral Zn and Fe levels in whole blood before and after antioxidant supplementation.
| Group 1 | Group 2 | Group 3 | |||
| Before supplementation | After supplementation | Before supplementation | After supplementation | ||
| GSH (μmolmL−1) | 1.47±0.28 | 0.78±0.06a | 3.55±0.83a,b | 0.58±0.06d | 1.49±0.16c |
| Vit. E (μmolmL−1) | 22.00±0.74 | 14.41±1.13a | 22.83±3.30 | 21.35±0.71d | 33.82±1.22a,c |
| Fe (μgmL−1) | 886.25±86.41 | 802.22±32.35 | 735.93±43.24 | 723.05±26.65 | 674.43±16.24 |
| Zn (μgmL−1) | 11.93±0.62 | 11.34±0.94 | 13.89±0.69 | 11.65±0.43 | 14.21±0.62 |
All values are mean±SEM; n=12 (group 1), n=9 (group 2) and n=11 (group 3).
Before the antioxidant supplementation, plasma concentrations of vitamin E decreased in group 2 in relation to groups 1 and 3 (Table 5). After the antioxidant supplementation significant increases were detected in patients from groups 2 and 3. No significant changes in zinc and iron concentrations were detected among the different groups before and after the antioxidant supplementation (Table 5).
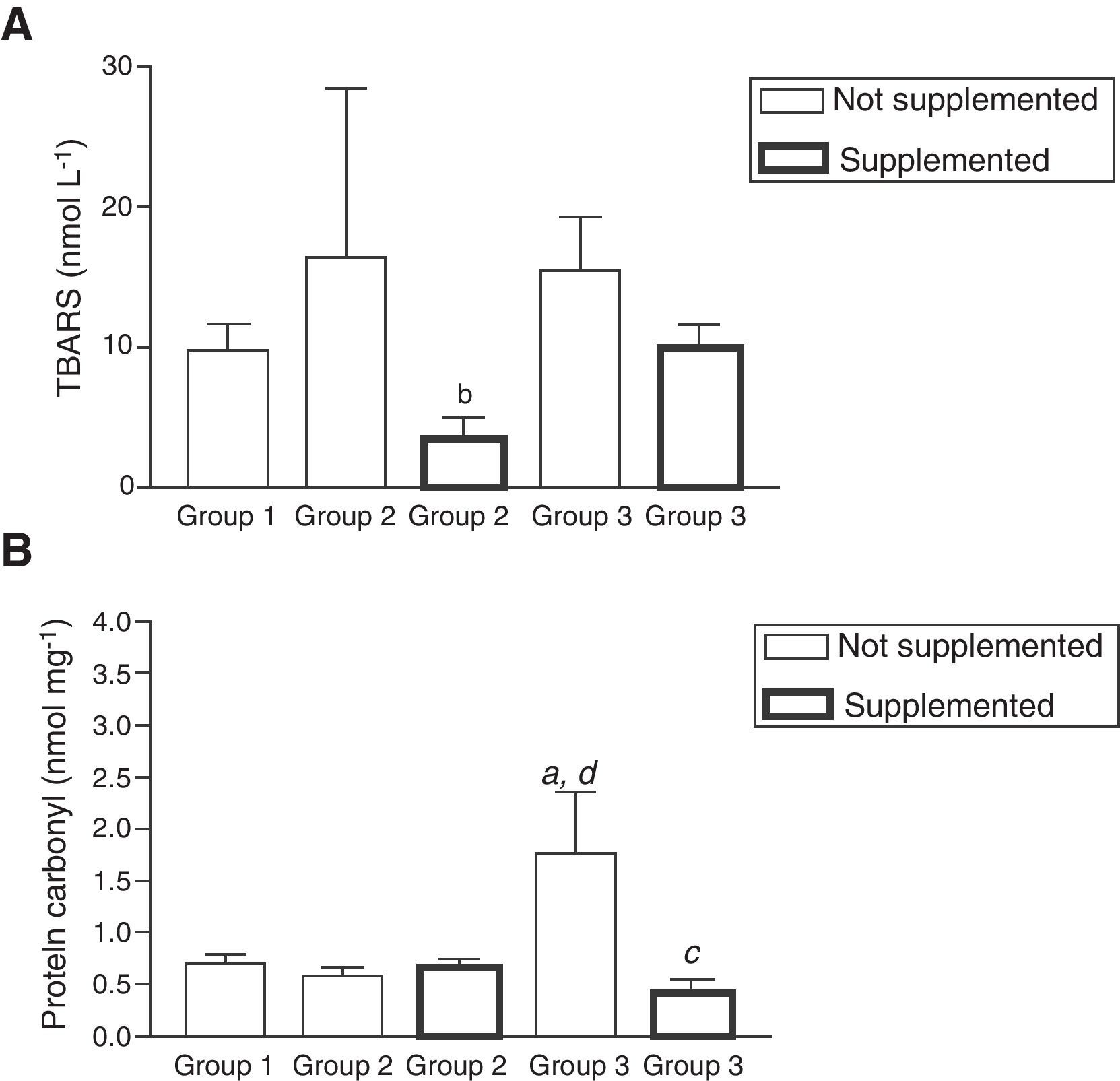
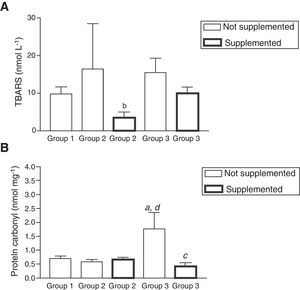
No significant changes in TBARS concentrations were observed before the antioxidant supplementation, however after this intervention a significant decrease was observed in patients belonging to group 2 (p<0.01; Fig. 2A). Contrary to the results revealed for TBARS levels before supplementation, there was a significant increase in the levels of protein carbonyls in patients from group 3 compared to groups 1 and 2 (p<0.01) (Fig. 2B). After the antioxidant supplementation, a significant decrease in these concentrations in the same group (p<0.01) was observed (Fig. 2B).
Oxidative stress markers evaluated as levels of lipoperoxidation (TBARS) (A) and protein carbonyl (PC) (B) before and after antioxidant supplementation. Note: (a) Statistical differences compared to healthy patients (p<0.01); (b) between supplemented and non-supplemented patients infected HCV (p<0.01); (c) between supplemented and non-supplemented patients treated with antiviral therapy (p<0.01); (d) between non-supplemented patients treated with antiviral therapy compared to non-treated patients infected HCV (p<0.01). All values are mean±SEM; n=12 (group 1), n=9 (group 2) and n=11 (group 3).
Related studies on HCV patients have already been reported2,4,26 revealing similar results to those found in the present study, all of them indicating a condition of a systemic oxidative stress associated with the disease. Increased enzymatic activities of SOD (88.3%) and CAT (31.2%) together with a markedly decreased in GR (77.1%) activity, as well as depletions of GSH (46.9%) and vitamin E (34.5%) contents were found in untreated HCV patients compared to controls. A similar profile was found in patients treated with pegylated interferon associated with ribavirin compared to untreated HCV patients, in which increased CAT (36.0%), GPx (7.8%), and GST (365.3%) activities, increased PC (56.5%) concentrations and a persistent GSH depletion (25.6%) were detected. Before the antioxidant supplementation the increased activities of SOD (88.3%) and CAT (31.2%) found in the red cells of untreated HCV patients probably reflect an antioxidant compensation regarding the virus infection per se. Also, the marked increases found in the activities of GR (444.4%) and in the concentrations of PC (56.5%), when comparing treated with untreated HCV patients, could be attributable to an antioxidant response regarding the oxidative injury caused by the antiviral treatment. Such an increased SOD response is in accordance to that reported in the blood of HCV patients in related studies.6,27 After supplementation SOD activity remained unchanged, a response that could be associated with the ability of Zn acting as an acceptor of electrons, which is able to scavenge superoxide anions in its free form.28 After supplementation the previously enhanced CAT activities found in both supplemented groups showed a strong decrease (41.2% and 67.8%, respectively), also suggesting an antioxidant compensation regarding the viral infection per se and/or the antiviral treatment.
A significant increase of GPx activity was observed in groups 2 (21.91%) and 3 (31.46%) compared to controls. Similar to the CAT response after supplementation, GPx activity in group 3 was also decreased (33.1%), suggesting the chain-breaker ability of vitamin E in attenuating lipid peroxidation processes.7
A marked decrease was detected in GR activity in untreated HCV patients compared either to controls or to treated patients (77.14%; 83.67%, respectively). Before supplementation a remarkable increase (512.5%) was detected in GR activity between groups 2 and 3 (p<0.01), which also might be attributable to a compensatory mechanism against the oxidative stress promoted by the presence of HCV and the antiviral treatment, respectively. After the antioxidant intervention the decrease (85.3%) found in GR activity of patients from group 3 might again reflect such antioxidant compensation.
Before supplementation GST activity showed a marked increase in G3 compared to G2 (361.2%), also suggesting that the antiviral therapy exacerbated the oxidative insult in those patients. After the antioxidant intervention a significant decrease in GST activity was observed in supplemented groups 2 and 3 (86.2% and 96.0%, respectively), coinciding with the decreased CAT and GR activities, decreased contents of protein carbonyls, as well as the recovery of GSH contents. As GST is also involved in the elimination of hydroperoxides, the decline in TBARS levels in supplemented subjects from group GII might be related to such decrease found in GST activity.
Before supplementation, a significant decrease in GSH concentrations was found in patients from supplemented groups compared to controls (40.9% and 56.2%, respectively). After antioxidant supplementation highly significant increases in GSH contents in supplemented groups (354.15% and 157.2%, respectively) were found, suggesting a synergistic interaction between vitamin E and GSH,7 as well as a possible indirect antioxidant action of Zn.29 This metal increases the biosynthesis of GSH via the enzyme glutamate cysteine ligase,30 being also able to protect non-toxic induction of oxidative stress caused by H2O2.31 Depletion of plasma and erythrocytic Zn contents in chronic HCV patients compared to healthy subjects was already reported.27,32 After antioxidant supplementation Zn (as well as iron) contents remained unchanged in both supplemented groups thus suggesting that part of Zn could be driven to GSH biosynthesis, since its concentrations increased significantly in both groups. In agreement with these results, Murakami et al.33 found that after supplementation with zinc, vitamins C and E for 24 weeks in patients with hepatitis C antiviral treatment and without treatment, total levels of Zn concentrations measured in whole blood remained unchanged. Although HCV patients revealed a sufficient dietary vitamin C intake, the dietary intake of vitamin E and Zn presented low levels (Table 2).
Before antioxidant supplementation depletions of vitamin E were found in group 2 compared to groups 1 and 3 (p<0.01). The maintenance of vitamin E concentrations in group 3 might be due to decreased viral replication caused by the antiviral treatment, helping to maintain integrity of the hepatic tissue, thus preserving the function of the α-tocopherol protein carrier found in the hepatocytes.34 After the antioxidant supplementation a significant increase was detected in patients from group 3 (p<0.05), in parallel to the increase of GSH levels and also to the attenuation of lipid peroxidation and protein oxidation. In this context, vitamin E doses of 800mg or more were effective in suppressing oxidative stress in humans,35 in accordance with the dose used in this study.
In agreement with other related studies,36,37 although no significant, TBARS concentrations showed a strong tendency of increase (97.28%) in HCV patients, while after the antiviral treatment a strong tendency of decrease (47.4%) was found in group 3. Contrasting to these results, PC values showed an increase (56.5%, p<0.05) in patients of group 3 compared to group 2, similarly to the finding of a related study.36 After antioxidant supplementation no changes in TBARS concentrations in group 3 were found, while in group 2 a significant decrease (88.74%; p<0.01) was detected. This response could be a consequence of the antioxidant action of vitamin E, including a synergistic action with vitamin C in protecting lipid membranes from oxidative insult, since TBARS contents in group 3 after supplementation were similar to those of group 1, a result corroborated by a related study.37
After the antioxidant intervention, no significant change in PC levels in patients from group 2 was detected. However, in group 3 there was a significant decrease (76.3%; p<0.01), suggesting a possible action of zinc in binding to sulfhydryl groups of proteins, thereby stabilizing and decreasing its reactivity with transition metals to generate pro-oxidants.28
No significant changes in AST concentrations among the three groups were observed. Unlike AST content, there was a significant increase in ALT and GGT contents between HCV patients and controls, in accordance with a related study.38 This confirms that in chronic hepatitis C elevations of ALT are more frequent than AST levels, probably due to the fact that ALT is found primarily in the cytoplasm and in the parenchyma of hepatocytes, thereby being a better marker of liver disease.39 A similar study detected AST and ALT increases before antiviral treatment whereas after such treatment significant decreases were found, suggesting that the action of antiviral therapy attenuated HCV replication, therefore decreasing the injury to liver tissue.2 This response was only applied to ALT levels in the present study, AST levels remaining the same before and after treatment, which is corroborated by a related study.40 After 6 months of supplementation no changes in AST and GGT concentrations were found. In contrast, a similar study in patients with hepatitis C submitted to antiviral treatment and supplementation with zinc, vitamins C and E for 24 weeks showed an increase in the concentration of ALT and AST with antiviral therapy and a significant decrease after antioxidant supplementation.35
In summary, despite that increasing ROS levels in HCV patients might be beneficial by suppressing HCV replication,5 untreated HCV patients exhibited an oxidative stress condition probably caused by the virus infection. This oxidative insult was characterized by GSH depletion, increased contents of protein carbonyl together with the upregulation of antioxidant enzymes such as CAT, GST and GPx. Therefore, it is here suggested that the treatment by pegylated interferon associated with ribavirin might be responsible for exacerbating such oxidative challenge in HCV patients.
Finally and more importantly, despite that ROS overgeneration is probably a marginal fact in the pathogenesis of HCV infection after clearing HCV with antivirals, the concomitant treatment using vitamin E and C and Zn conferred an antioxidant protection to HCV patients both to untreated and treated with interferon combined with ribavirin. This combination of antioxidant supplementation attenuated blood processes of protein oxidation, increased GSH contents and decreased the activities of the antioxidant enzymes GPx, GR, GST and CAT.
Conflict of interestThe authors declare no conflict of interest.
D. Wilhelm Filho (Proc. 303951/2009-5) and R. C. Pedrosa (Proc. 300718/2003-9) are recipients of CNPq research scholarships.