Hepatocellular carcinoma (HCC) is one of the major malignancies worldwide and its incidence is on the rise, closely related to advanced liver disease. Sorafenib chemotherapy is one of the main treatment options for patients with advanced HCC. Despite several reports on HCC multidrug resistance, the underlying regulatory mechanisms are still unclear. In this study, we found circ-001241 was significantly upregulated in HCC tissues and cells. Knockdown of circ-001241 markedly inhibited HCC cell proliferation and decreased sorafenib-resistance. More importantly, circRNA acts as a ceRNA to suppress the expression and activity of miR-21-5p, leading to the increase in TIMP3 expression. In addition, circRNA-001241 facilitated HCC sorafenib-resistance by regulating the miR-21-5p/TIMP3 axis. Taken together, our study elucidated the oncogenic role of circ-001241 in mediating sorafenib resistance in HCC, providing insights and opportunities to overcome sorafenib resistance in patients with advanced hepatocellular carcinoma.
El carcinoma hepatocelular (CHC) es una de las principales enfermedades malignas en todo el mundo y su incidencia va en aumento, estrechamente relacionada con la enfermedad hepática avanzada. La quimioterapia con sorafenib es una de las principales opciones de tratamiento para los pacientes con CHC avanzado. A pesar de varios informes sobre la multirresistencia del CHC, los mecanismos reguladores subyacentes aún no están claros. En este estudio encontramos que circ-001241 estaba significativamente regulado en los tejidos y células del CHC. El knockdown de circ-001241 inhibió notablemente la proliferación de las células del CHC y disminuyó la resistencia al sorafenib. Más importante aún, el circRNA actúa como un ceRNA para suprimir la expresión y la actividad de miR-21-5p, lo que conduce al aumento de la expresión de TIMP3. Además, circRNA-001241 facilitó la resistencia a sorafenib del CHC, mediante la regulación del eje miR-21-5p/TIMP3.En conjunto, nuestro estudio dilucidó el papel oncogénico de circ-001241 en la mediación de la resistencia a sorafenib en el CHC, proporcionando conocimientos y oportunidades para superar la resistencia a sorafenib en pacientes con carcinoma hepatocelular avanzado.
Hepatocellular carcinoma (HCC) is a representative type of liver cancer1. Due to its high recurrence rate, increasing incidence, and poor prognosis, HCC has become a global health challenge. It has been suggested that it is estimated that up to one million people will be affected by liver cancer each year by 2025.2 Although various known risk factors persuading HCC have been explored, little is known about the potential molecular approaches.
In the treatment of advanced HCC, multikinase inhibitors and sorafenib have shown significant clinical efficacy.3 However, because many patients may develop acquired resistance to sorafenib, new targets are needed to reduce drug resistance. In recent years, immunotherapy can be used in combination with drugs currently used to treat hepatocellular carcinoma to produce a combined/synergistic effect.4 Clinical trials of monoclonal antibodies (mAbs), ramucirumab, which targets vascular endothelial growth factor(VEGF)receptor-2.5 and bevacizumab,6 which inhibits VEGF receptor binding, with chemotherapy, immunotherapy, or other agents used in cancer treatment are currently underway, adding new therapeutic options to the field of advanced hepatocellular carcinoma treatment.
As a new class of endogenous ncRNAs, circRNAs are related to tumorigenesis and have become the focus of clinical research in recent years. They form covalent, closed-loop structures through specific splicing and are considered to be the main subtype of gene transcription, which differs from the linear RNA terminated by 5ʹ hat and 3ʹ poly (A).7,8 Stably expressed circRNAs almost present in all types of species9 and are not affected by RNA exonuclease.10 CircRNA is involved in various biological processes, including HCC cell proliferation, apoptosis and metastasis. Therefore, CircRNAs are suitable as diagnostic biomarkers for tumors, including liver cancer.11 In liver cancer, some studies have also revealed the presence of a large number of circRNAs, such as circMET and circFOXM.12,13 However, the mechanism and function of circRNAs involved in sorafenib resistance in liver cancer still need to be further explored.
In this experiment, GSE datasets (GSE94508) taken from Gene Expression Omnibus database (GEO, http://www.nih.gov/geo) were analyzed by the GEO2R online tool. The results showed that circRNA-001241 was amplified and expressed in HCC samples compared with normal samples. Further identified that circRNA-001241(has-circ-0000508) was preserved and suggestively increased in sorafenib-resistant HCC tissues. Studies on gain and loss of function have shown that circRNA-001241 stimulates the growth of cancer cells in vivo and in vitro. In addition, we also found that circRNA-001241 may act as a sponge for miR-21-5p and up-regulate the level of TIMP3, thus supporting drug resistance of HCC. We found new insights into monitoring mechanisms of circRNA-001241 in tumorigenesis and sorafenib resistance in HCC.
Materials and methodsTissue samplesFresh adjacent normal tissue (ANT) and HCC tissues samples were collected from 56 HCC patients (Department of Hepato-Biliary Surgery, Guizhou Provincial People's Hospital) between 2019 and 2020 and stored in liquid nitrogen till usage. The investigation procedure was approved by Ethics Committee of the Guizhou Provincial People's Hospital, and written informed consent from each patient was obtained preceding surgery.
Cell culture and transfectionNormal liver cell lines LO2, Huh-7 and hepatocellular carcinoma cell lines HepG2 were purchased from type I culture library cell bank of Chinese Academy of Sciences. Cells were cultured using DMEM and RPPI-1640 (Gibco, Carlsbad, CA, USA), 10% fetal bovine serum (Gibco) in an incubator at 37°C, 5% CO2, and 100% humidity. HepG2-SR and Huh7-SR cells were prepared using previously described procedure.14
Cells (2×105cells/well) were seeded onto 6-well dishes at 24h and then transfected with control or experimental siRNA for circRNA_001241 or si-TIMP3 per dish at 80% confluence using the Lipofectamine 3000 (Life Technologies, Shanghai, China) according to the manufacturer's instructions. The primers of si-circRNA-1 were 5ʹ-GTGTGGCTATGGAGGTCACGT-3ʹ; si-circRNA-2 were 5ʹ-GTGGCTATGGAGGTCACGTCC-3ʹ;the primers of si-TIMP3 were 5ʹ-GGACTGTGGTTACTGTCAT-3ʹ.
Preparation of RNA and quantitative real-time PCRTotal RNA was extracted from HCC tissue samples employing TRIzol Reagent (Invitrogen). RNase R treatment was performed using 3U/mg RNase R (Epicenter) at room temperature (37°C) for 15min. RNA (1μg) was retrotranscribed with RT Master Mix (Prime Script-Takara, Japan) and Random or Oligo (DT) primers for real-time quantitative PCR (RT-PCR). Reverse transcription of miRNA and reverse transcription of mRNA into cDNA were completed respectively. The cDNA was amplified using a common SYBR Green Master Mix (Roche, Mannheim, Germany). CT values were measured throughout the exponential growth period. The relative gene expression was measured by 2-△CT. RiboBio (Guangzhou, China) designed a large ring miRNA quantitative RT-PCR primer set, with one RT primer pair per set and one qPCR primer pair per set) for accurate use of miR-21-5p. The relative expression of miR-21-5p was normalized as human U6 snRNA. The primers used for RT-PCR were: circRNA-001241 primers were 5ʹ-GCTGTGGAGGTCGCTGTGTGG-3ʹ (sense) and 5ʹ-AGACCTTCAGA GCCACATGGATG-3ʹ (anti-sense); TIMP3 primers were: 5ʹ-CAAGATGCCCCATGTGCAGT-3ʹ (sense) and 5ʹ-TCTCCACGAAGTTGCACAGC (anti-sense); GAPDH primers were 5ʹ-ACAACTTTGGTATC GTGGAAGG-3ʹ (sense) and 5ʹ-GCCATCACGCCACAGTTTC-3ʹ (anti-sense). U6 primers were 5ʹ-CAAGGATGACACGCAAA-3ʹ (sense) and 5ʹ-TCAACTGGTGTCGTGG-3ʹ (anti-sense).
Plasmid and siRNA construction and cell transfectionThe circRNA-001241overexpression plasmid, circRNA-miRNA and TIMP3-miRNA binding mutation plasmid were attained from Sangon Biotech (Shanghai, China), full-length circRNA-001241 cDNA was synthesized and cloned into an overexpressed vector pLO-ciR containing a front and back circular frame to support RNA circalization. CircRNA-001241 and siRNA of miR-432-5p inhibitor, mimic, and negative control were synthesized from GenePharma (Shanghai, China). Plasmid transfection was performed using Lipofectamine 3000 (Invitrogen, CA, USA). The miRNA and siRNA analogs were transfected into cells using RNAIMax (Invitrogen) according to the manufacturer's guidelines. miR-21-5p primers were: 5ʹ-GCGCGTAGCTTATCAGACTGA-3ʹ (sense) and 5ʹ-AGTGCAGGGTCCGAGGTATT(anti-sense).
Actinomycin D and RNase R treatmentActinomycin D (2mg/mL) or DMSO (Sigma–Aldrich, St. Louis, MO, USA) was added as control to the medium to inhibit transcription. Total RNA (5mg) was cultured at room temperature for 30min with or without 3U/mg RNase R (Epicenter Technologies). The resulting RNA was then washed using the RNEasy Minelute cleaning kit (Qiagen, Germany). After the above treatment, RNA was transcripted into cDNA, and the expression levels of GAPDH and circRNA-001241 were observed by real-time fluorescence quantitative PCR.
Cell fractionation assayNuclear and cytoplasmic RNA was obtained using nuclear and cytoplasmic RNA purification kits (Invitrogen, CA, USA). The cells were collected and incubated with lysate on ice for 10min, then centrifuged at 12,000×g for 3min. Nuclear RNA was extracted from the nuclear bulb using the endogenous U6 micronuclear RNA as the control, and cytoplasmic RNA was extracted from the supernatant using GAPDH as the endoplasmic control.
Cell proliferation assayCCK-8 detection (Bimake, Shanghai, China) uses a similar method, as described earlier. Transfected cells were inoculated into 96-well plates (2×103cells/well) and incubated overnight. The cells were then treated with different concentrations of sorafenib for 48h. Add CCK-8 reagent 10mL to each well, and incubate at 37°C for 2h. The absorption value was measured at 450nm with a microplate analyzer.
Colony formation assays and apoptosis assayCells were inoculated into 6-well plates and treated with sorafenib for 48h. Cells treated with 1×103 were cultured in 6-well plates for 2 weeks. Paroxyformaldehyde (4%) was fixed and cell colonies were counted by crystal violet staining. For apoptosis, the cells were inoculated in plates containing 3×105cells/well and 6-well. Treat with sorafenib for 48h. Cells were collected and treated with PI staining solution and Annexin V-FITC under dark conditions for 15min. Flow cytometry (Gallios, Beckman, USA) was used to detect the proportion of cell apoptosis, and FlowJo 7.6.1.2.9 was used to analyze the proportion of cell apoptosis.
Biotin RNA pull down assayBiotin-labeled circRNA-001241 and NC probes were manufactured by the GeneChem Company. The cell lysate was incubated with strepavidin-coated magnetic beads (Invitrogen Carlsbad, USA) to pull down the biotin-coupled RNA complex according to the manufacturer's guidelines. The miRNA enrichment in the captured fractions was evaluated by qRT-PCR analysis.
Luciferase reporter assayThe circRNA-001241 or 3’UTR TIMP3 fragment was cloned full-length into the dual luciferase reporter vector psicheck-2 (Promega, WI, USA) to construct the luciferase reporter vector from the potential binding site mutation of miR-21-5p. The above MUT or WT plasmids were co-transfected with miR-21-5p mimics or miR-NC. Dual-luciferase reporter assay system (Promega, Madison, WI, USA) was used to evaluate luciferase activity 48h after incubation. The experiment was repeated, and the comparison luciferase activity was normalized to luciferase from the kidney as the internal control.
Western blottingThe protein was extracted by kit method (total protein extraction kit – Thermo Fisher Scientific, MA, USA). The total protein lysate was separated by SDS-polyacrylamide gel electrophoresis and transferred to PVDF membrane. The membrane was incubated overnight at 4°C using a primary antibody that recognized TIMP3 (1:1000 dilution Abcam), β-actin (1:10,000 dilution; Proteintech) in the United States. After incubation with secondary antibody (diluted at 1:5000; Abcam), chemiluminescent protein bands were observed using the GE Amersham Imager 600(GE Healthcare, USA).
Tumor xenograft modelIn this experiment, BALB/c nude mice aged 6 weeks were purchased from Hunan SJA Experimental Animal Co., Ltd., China. Each mouse was injected subcutaneously 6×107 HepG2-SR cells transfected with si-circRNA-001241 or si-NC suspended in 100mL of Hanks’ balanced salt solution. During 6 days of injection, mice were intraperitoneally injected with PBS or sorafenib (20mg/kg) once a day for 4 weeks. According to the treatment, the tumors on the mice were actually divided into the following groups: group 1, si-circ-1transfected cells; group 2, si-circ-1 transfected cells+sorafenib; group 3, si-NC transfected cells+sorafenib; the tumor size in each animals was measured every 3 days. On day 21, the animals were killed and their tumors collected. Tumor tissues were fixed with 4% formalin and frozen at −80°C. To evaluate tumor metastasis, sorafenib 20mg/kg or PBS was injected through the tail vein. At 48h after administration, fluorescence intensity in each group was measured on the Xenogen IVIS lumina XR imaging system from Caliper Life Science (USA). The mice were then euthanized, the tumor and metastatic tissue were collected, and in vitro imaging was performed. The tumor tissues were made into frozen sections and observed under fluorescent microscope. The fixed tissues were paraffin-embedded and sectioned for H&E staining and immunohistochemical staining.
The animal experiments were approved by the Animal Care Committee of Guizhou Provincial People's Hospital. All experiments involving mice were conducted in accordance with the guidelines for animal welfare formulated by the laboratory animal center at Guizhou Provincial People's Hospital.
Statistical analysisData were assessed using Student's t-test in SPSS 18.0 and expressed as mean±SD. Kaplan–Meier analysis was used to decipher the association between circRNA-001241 expression and patient survival (*P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001).
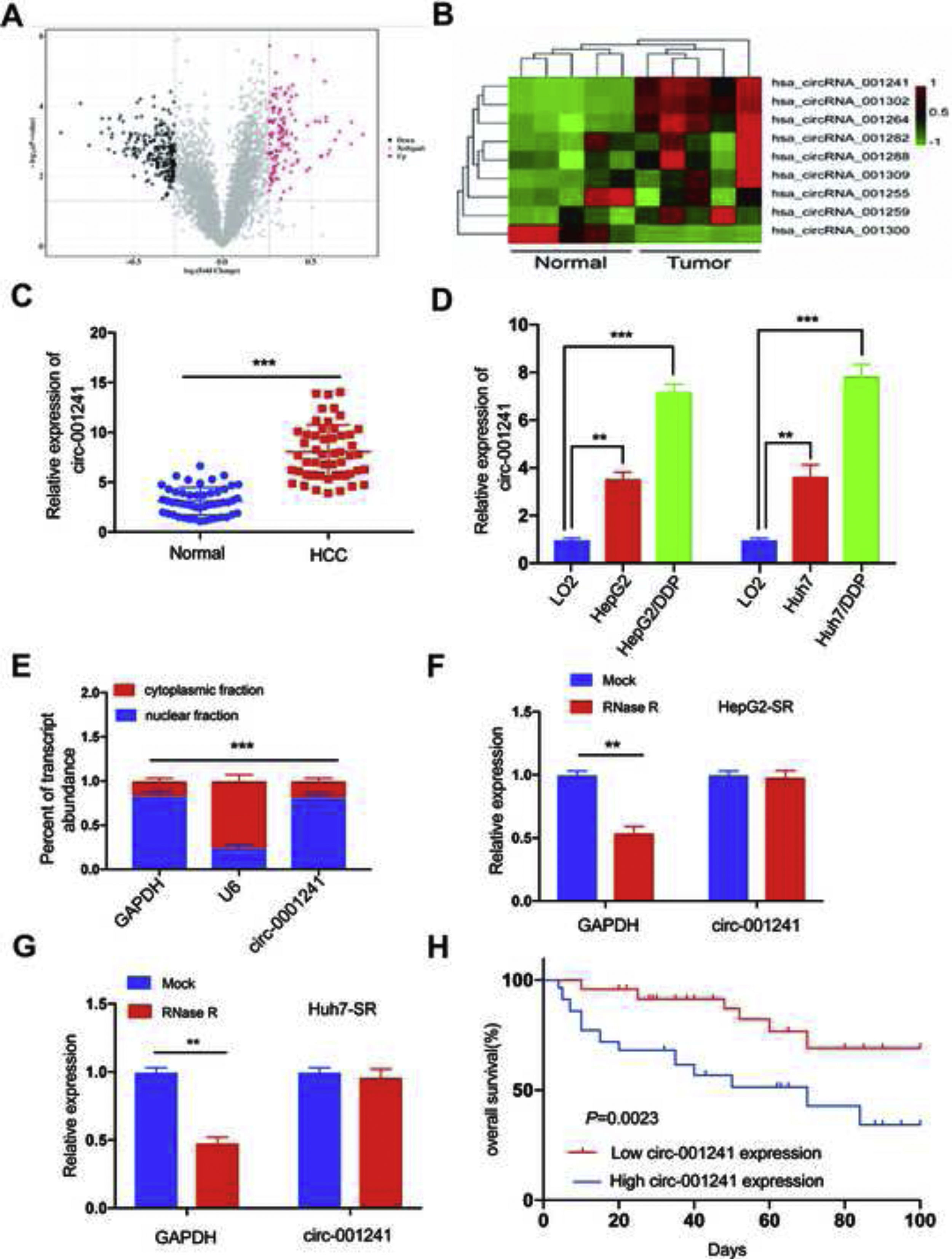
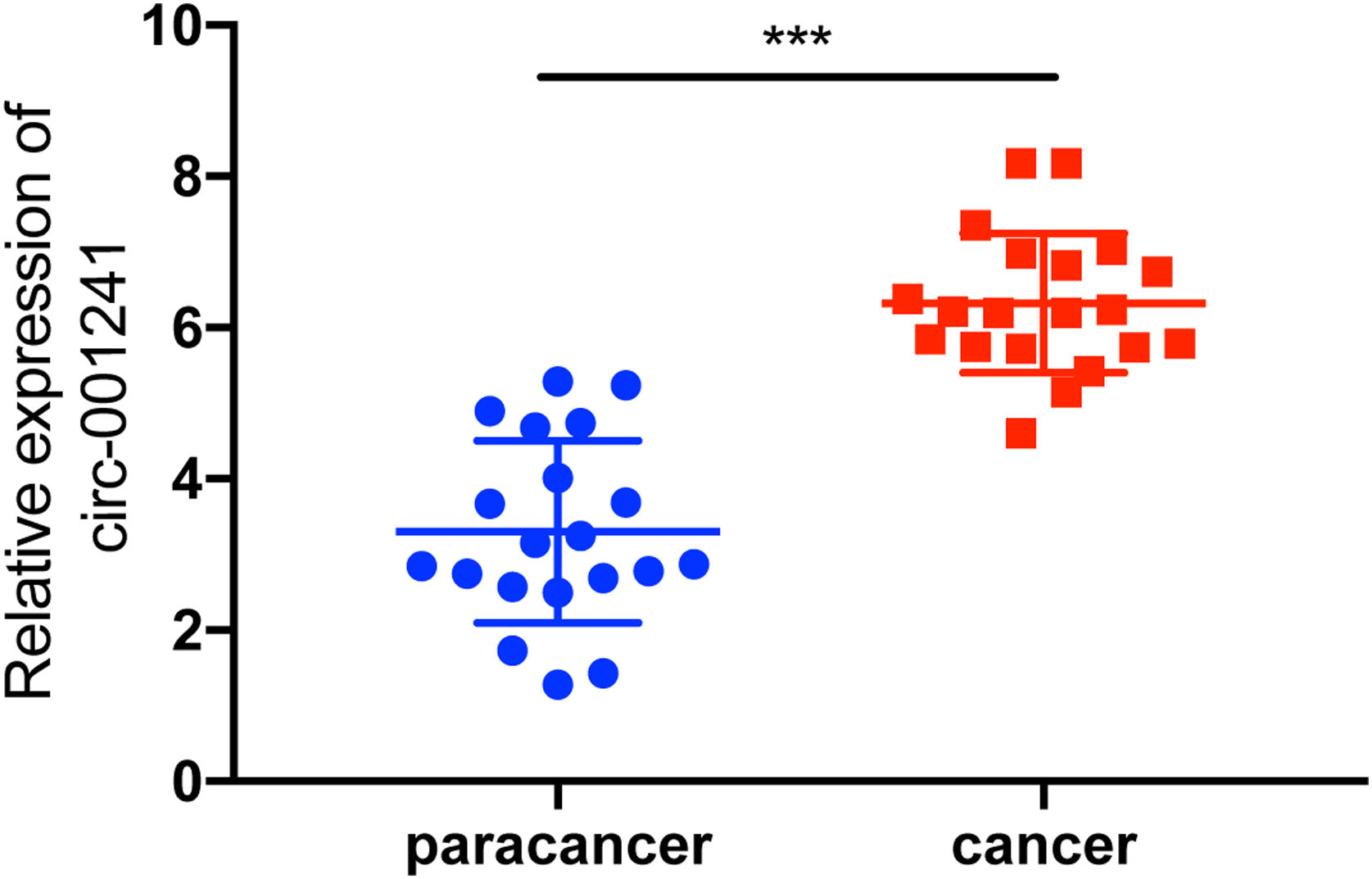
ResultsUpregulated circRNA-001241correlates with sorafenib resistance and poor prognosisCircRNA-001241 exhibited high relative fold change in GSE datasets. Therefore, circRNA-001241 was selected for further study (Fig. 1A). The relative expression of circRNA-001241 was detected in HCC patients with RT-PCR. The expression of circRNA-001241 was significantly upregulated in primary HCC samples comparative to the compared with particular healthy adjacent tissues (Fig. 1B). In patients with cirrhosis developing into hepatocellular carcinoma, the expression of HCC tissues was higher than that of paraneoplastic tissues, and the difference was significant (Supplementary Figure 1), and the same upregulated trend was confirmed in HepG2-SR and Huh7-SR cells compared with adjacent normal tissue (Fig. 1C). Then, the comparative expression levels of circRNA-001241 were observed in cytoplasm and nucleus of HepG2-SR cells (Fig. 1D). The RT-PCR results showed that circRNA-001241 was augmented in cytoplasm. Additionally, the linear form of circRNA-001241fragments remained following RNase R treatment (Fig. 1E and F). Moreover, We also analyzed the clinical characteristics and pathological stages of HCC patients, and the results showed that the increase of circ-001241 in HCC tissues was significantly associated with larger tumor size and higher TNM stage in HCC patients. (Table 1). Importantly, Kaplan–Meier analysis showed that high expression of circRNA-001241 is associated with inferior overall endurance (Fig. 1G) and recurrence-free survival (Fig. 1H) compared with low circRNA-001241 expression. These results suggested that the overexpression of circRNA-001241 may be complex in the process of tumorigenesis and multidrug resistance in HCC.
Circ-001241 is upregulated in HCC and associated with sorafenib resistance and poor prognosis. (A) The volcano plot shows deregulated circRNAs between normal tissue samples and HCC tissue samples from the GSE94508 datasets. (B) The heatmap showed the most differential circRNAs and circRNA_001241 is significantly increased in HCC tissues compared to normal tissues. (C) Comparison of circRNA_001241expression in 50 pairs of HCC specimens and adjacent normal tissues. (D) Compared with the parent cell lines (HepG2 and Huh7), the expression of circ-001241 in HepG2-SR and Huh7-SR cells was significantly increased. (E) Levels of circ-001241 in nuclear and cytoplasmic fractions of HepG2-SR cells. (F and G) RNA from HCC cells was treated with or without RNase R for RT-qPCR. The relative levels of circ-001241 were normalized to the values measured in HepG2-SR and Huh7-SR cells. (H) Kaplan–Meier curves in HCC patients with high (n=25) or low circ-001241expression (n=25) for overall survival. The results are presented as the mean±SD. *P<0.05, **P<0.01, ***P<0.001.
Correlation between circ-001241 Expression and Clinicopathologic Parameters in HCC patients (n=56).
| Parameters | Circ-001241 expression | P-value | |
|---|---|---|---|
| High (n=39) | Low (n=17) | ||
| Gender | 0.603 | ||
| Male | 21 | 10 | |
| Female | 18 | 7 | |
| Age, years | 0.364 | ||
| ≤60 | 23 | 13 | |
| >60 | 16 | 4 | |
| AFP, ng/mL | 0.428 | ||
| ≤400 | 11 | 6 | |
| >400 | 28 | 11 | |
| HBV DNA, cps/mL | 0.321 | ||
| ≤500 | 17 | 6 | |
| >500 | 22 | 11 | |
| Cirrhosis | 0.735 | ||
| No | 19 | 5 | |
| Yes | 20 | 12 | |
| Microvascular invasion | 0.454 | ||
| Absent | 21 | 10 | |
| Present | 18 | 7 | |
| Tumor size, cm | 0.038* | ||
| ≤3 | 10 | 4 | |
| >3 | 28 | 13 | |
| Tumor number | 0.239 | ||
| Single | 22 | 10 | |
| Multiple | 17 | 7 | |
| Tumor metastasis | 0.219 | ||
| Absent | 27 | 13 | |
| Present | 12 | 4 | |
| TNM | 0.017* | ||
| I–II | 18 | 11 | |
| III–IV | 21 | 6 | |
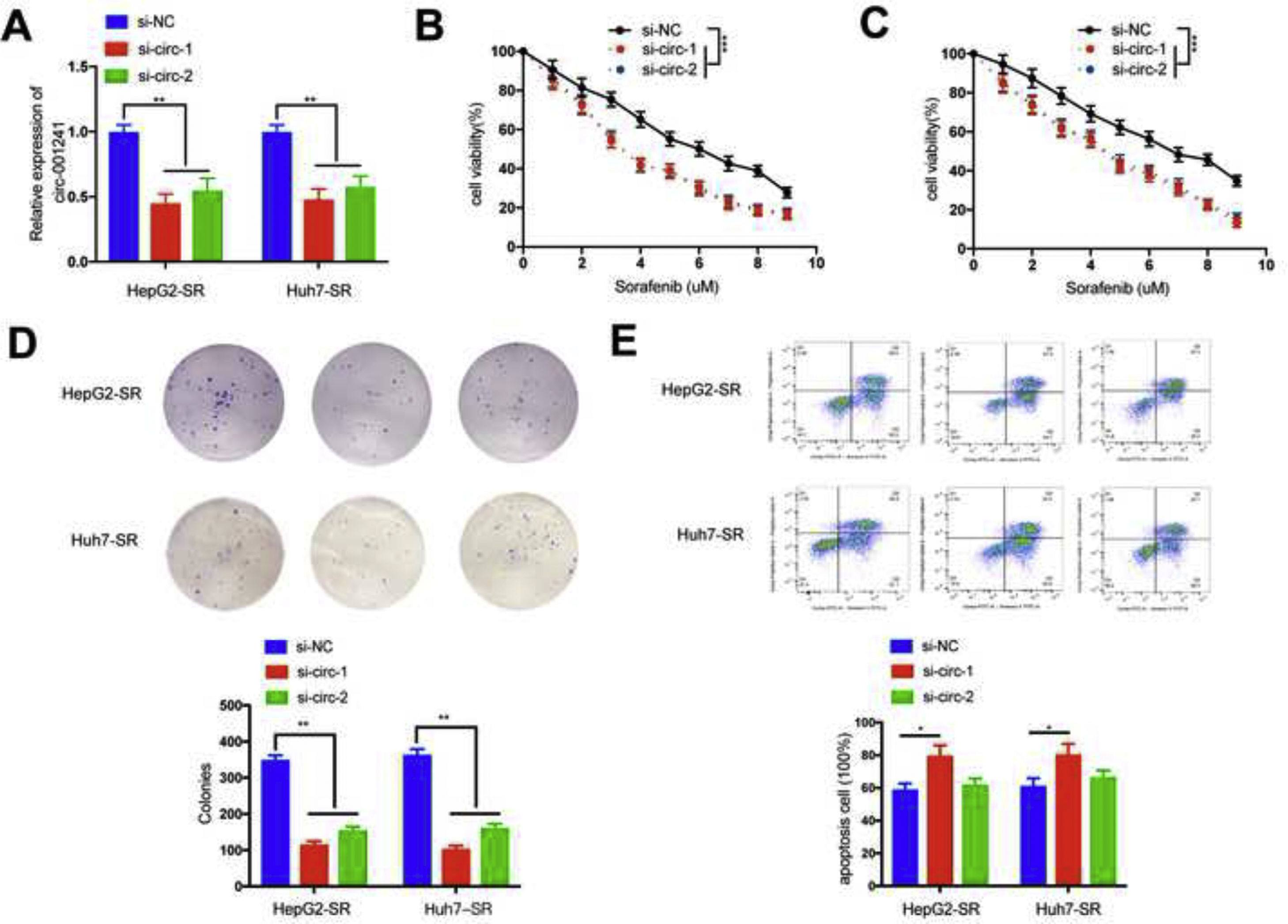
Firstly, two siRNA oligonucleotides (si-circ-1 and si-circ-2) were designed to target the exclusive back-splice connection of circRNA-001241; si-circ-1 effectively knocked down circRNA-001241 the lowest expression efficiency (Fig. 2A). Then, circRNA-001241 siRNA transfection reduced tolerance to sorafenib and cell viability in HepG2-SR and Huh7-SR cells. HepG2-SR and Huh7-SR cells transfected with circRNA-001241siRNAs were treated with sorafenib at different doses for 48h, respectively, and IC50 was obtained, indicating that circRNA-001241siRNAs transfected could give a warning of HCC cells to sorafenib (Fig. 2B and C). Additionally, circRNA-001241 knockdown expressively reduced the cell colonies (Fig. 2D) and promoted apoptosis after sorafenib (5μM) treatment for 48h (Fig. 2E). Collectively, these results showed that circRNA-001241 siRNAs transfection might prepare HCC cells to sorafenib administration by moderated propagation, tumorigenesis and increased apoptosis.
Downregulation of circ-001241 facilitates sorafenib sensitivity of HCC cells. (A) Real-time PCR analysis of circ-001241 in HepG2-SR and Huh7-SR cells transfected with circ-001241 siRNAs (si-circ-1, si-circ-2) or negative control siRNA (si-NC). (B,C) HepG2-SR and Huh7-SR cells transfected with si-NC or circ-001241siRNAs were treated with different doses of sorafenib for 48h and analyzed with CCK-8 assay. (D) After treatment with sorafenib (5μm) for 48h, HepG2-SR and Huh7-SR cells were transfected with si-NC or circ-001241siRNA for colony formation test. (E) The apoptotic rates of HepG2-SR and Huh7-SR cells transfected with si-NC or circ-001241siRNAs after sorafenib (5μM) treatment for 48h were visualized using flow cytometry. The data was expressed as mean values±SD. *P<0.05, **P<0.01, ***P<0.001.
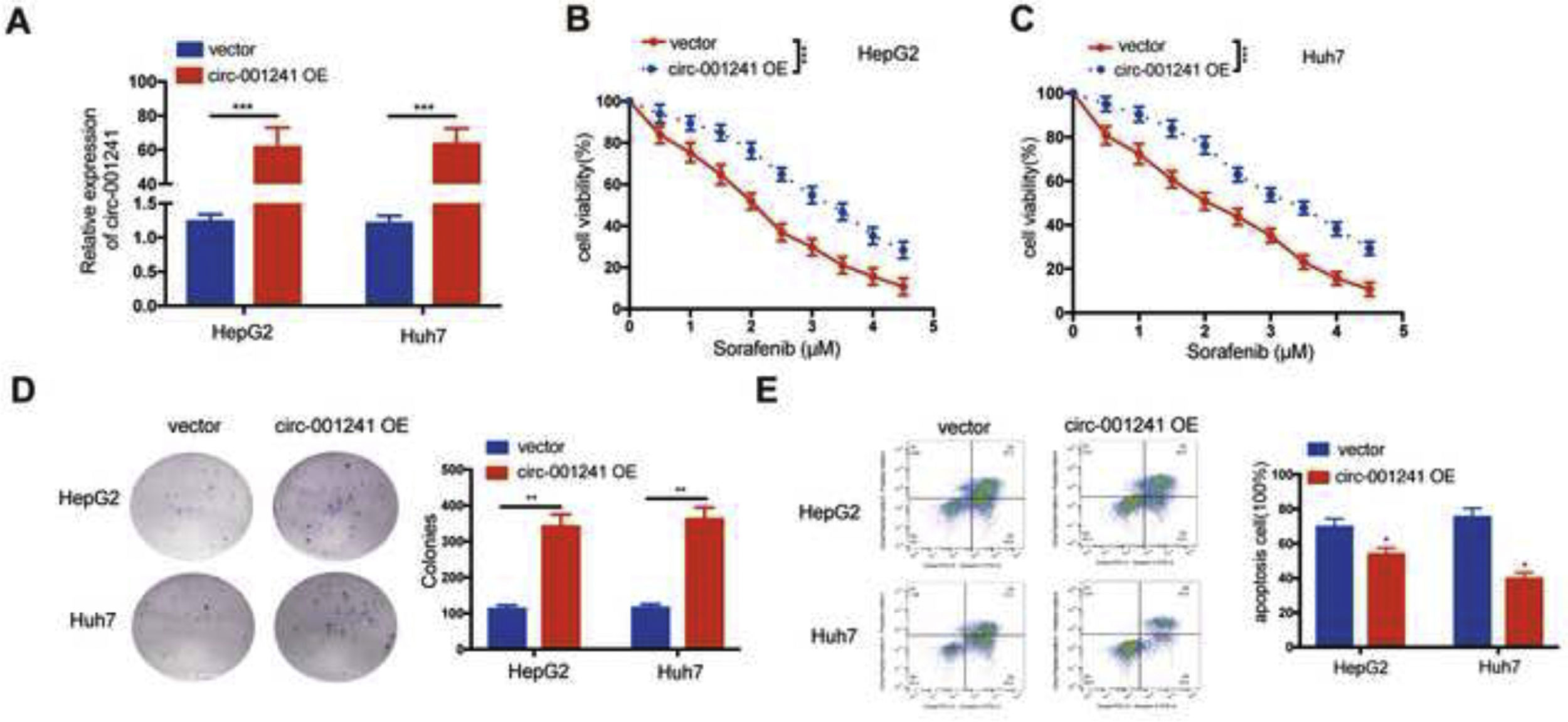
HepG2 and Huh7 cells were transfected with circRNA-001241 overexpressed plasmid (circRNA-001241 OE) to further evaluate the effect of circRNA-001241 on the sensitivity of sorafenib (Fig. 3A). The CCK-8 assay showed that circRNA-001241 OE might endorse tolerance of both HepG2 (Fig. 3B) and Huh7 (Fig. 3C) cells towards sorafenib treated with different concentrations. However, circRNA-001241 OE expression significantly increased the number of cell colonies and inhibited apoptosis in HepG2 and Huh7 cells after 48h treatment with sorafenib (2μM) compared with negative control (Fig. 3D and E). Collectively, data suggested that circRNA-001241 was pivotal in sorafenib sensitivity.
Overexpression of circ-001241promotes sorafenib resistance of HCC cells. (A) The expression level of circ-001241 in liver cancer cells stably transfected with circ-001241 or blank vector plasmid was detected by RT-qPCR. (B, C) Circ-001241 or vector transfected cells were treated with sorafenib at different doses, and the survival rate of cells was observed by CCK-8 method 48h later. (D) Colony formation assay of HCC cells transfected with circ-001241or vector after sorafenib (2μM) treatment for 48h. (E) The apoptosis levels in HCC cells transfected with circ-001241or vector after sorafenib (2μM) treatment for 48h were observed by flow cytometry. The data was shown as the mean±SD. **P<0.01, ***P<0.001.
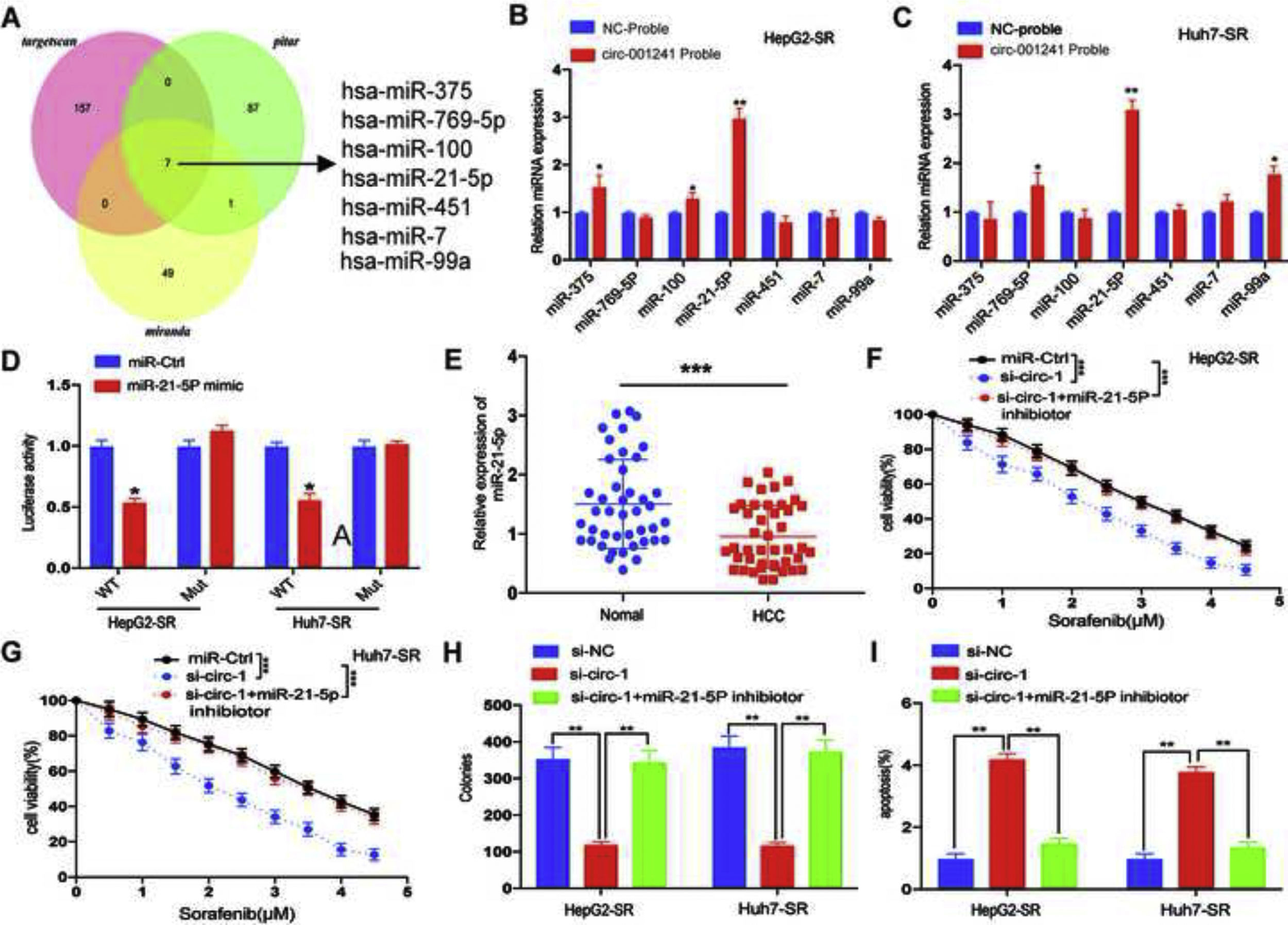
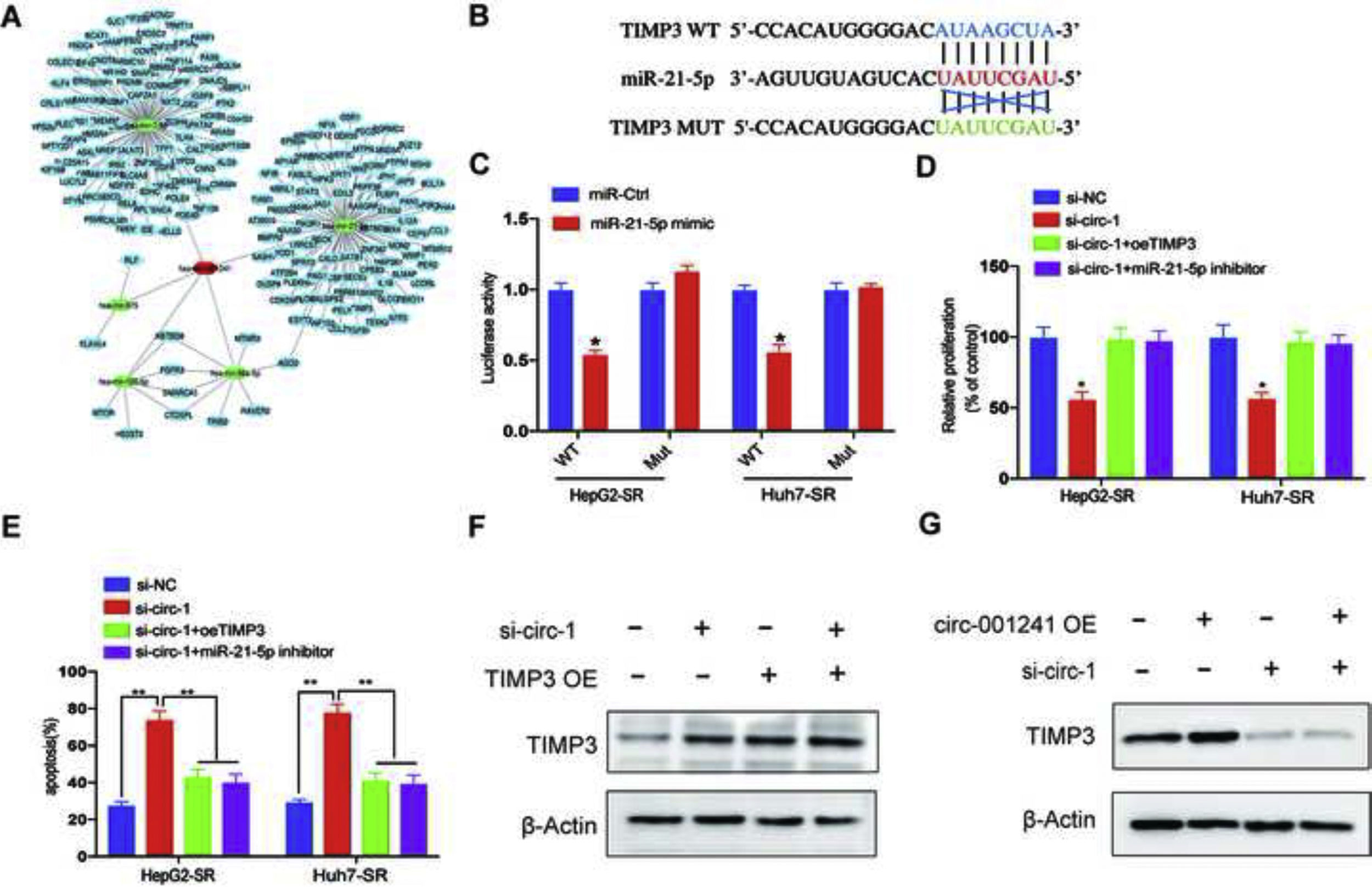
The prediction results of miRNA recognition elements in circRNA-001241 sequence were overlapping by using miRanda, Pitar and Targetscan, and a total of 7 candidate miRNAs were screened to determine whether circRNA-001241 might have spongy miRNAs in HCC cells (Fig. 4A). Then, miRNAs were extracted through the pull-down process and the levels of the seven candidate miRNAs were detected by RT-PCR. As shown in (Fig. 4B and C), in both HepG2-SR and Huh7-SR cells, miR-21-5p was abundantly pulled down by circRNA-001241. Next, the schematically constructed luciferase reporter vectors circRNA-001241 wild-type (circRNA-001241-wt) and circRNA-001241 mutated (circRNA-001241-mut). Luciferase reporter assay confirmed their complementary combinations (Fig. 4D). The result also proposed that miR-21-5p binds and subdues circRNA-001241 activity. In addition, miR-21-5p expression was decreased in HCC tissues compared with normal tissues (Fig. 4E), and then, after 5μm of sorafenib treatment, Co-transfection of si-circRNA-001241 with miR-21-5p inhibitor may save the proliferation and apoptosis of HepG2-SR cells and Huh7-SR cells (Fig. 4F and G) (Fig. 4H and I). Collectively, data indicated that circRNA-001241 persuaded sorafenib resistance was accelerated by the sponge effect on the expression of miR-342-5p.
MiR-21-5p is negatively regulated by circ-001241. (A) Schematic diagram of target miRNA overlap of circ-001241 predicted by miranda, pitar, and TargetScan. (B, C) The comparative levels of 7 miRNA candidates in HepG2-SR and Huh7-SR cells were detected by RT-qPCR. Several miRNAs were pulled down by circ-001241 and miR-21-5p was pulled down by circ-001241in both cell lines. (D) Luciferase reporter assay confirmed circ-001241 and miR-21-5p complementary combinations in both Huh7-SR and HepG2-SR cells. (E) Comparison of miR-21-5p expression in 50 pairs of HCC specimens and adjacent normal tissues. (F, G) The proliferation rates of HepG2-SR and Huh7-SR cells co-transfected with si-circ-1and miR-21-5p inhibitor were treated with different concentrations of sorafenib for 48h. (H, I) The colonies and apoptosis rates indicated that miR-21-5p inhibition rescues the decreased cell colonies and increased cell apoptosis due to inhibition of circ-001241in HepG2-SR and Huh7-SRcells with the sorafenib treatment (5μM) for 48h. The data was shown as the mean±SD. *P<0.05, **P<0.01, ***P<0.001.
To discover the possible existence of the circRNA–miRNA–mRNA axis in sorafenib-resistance, we predict potentially involved mRNAs rendering to the four algorithms (miRanda, RNAhybrid, miRWalk and TargetScan) prediction (Fig. 5A). The result exhibited that miR-21-5p can bind to 3ʹ-UTR region of TIMP3 (Fig. 5B), and luciferase reporter assay proved their complementary arrangements (Fig. 5C) and exposed that miR-21-5p binds and subdues the activity of TIMP3. In addition, after sorafenib treatment (5μM), transfection of TIMP3 overexpression plasmid (TIMP3 OE) or miR-21-5p inhibitor inhibited the proliferation of HepG2-SR and Huh7-SR cells and reduced sorafenib-induced apoptosis (Fig. 5D and E). To further observe the TIMP3 role in accelerating circRNA-001241 functions, a rescue assays TIMP3 overexpression and knockdown. These assays showed that TIMP3 could partially rescue the effect of circRNA-001241 (Fig. 5F and G), indicating that circRNA-001241 sustains sorafenib resistance by regulating the miR-21-5p/TIMP3 axis.
Circ-001241 facilitates HCC sorafenib-resistance by regulating the miR-21-5p/TIMP3 axis. (A) CeRNA network of circ-001241 with miRNA and their target genes. (B) The estimated binding sites of TIMP3 and miR-21-5p. (C) Luciferase reporter assay confirmed TIMP3 and miR-21-5p complementary combinations in HepG2-SR and Huh7-SR cells. (D, E) Proliferation and apoptosis rates of HepG2-SR cells and Huh7-SR cells transfected with a single transfection inhibitor or co-transfected with a specified vector 48h after sorafenib irradiation (5μm). (F, G) Western blotting was used to detect the expression level of TIMP3 in HepG2-SR cells transfected with si-circ-1 and TIMP3 OE or si-TIMP3 and circ-001241 OE. The data was represented as mean values as ±SD. *P<0.05, **P<0.01. TIMP3 OE: TIMP3 overexpression.
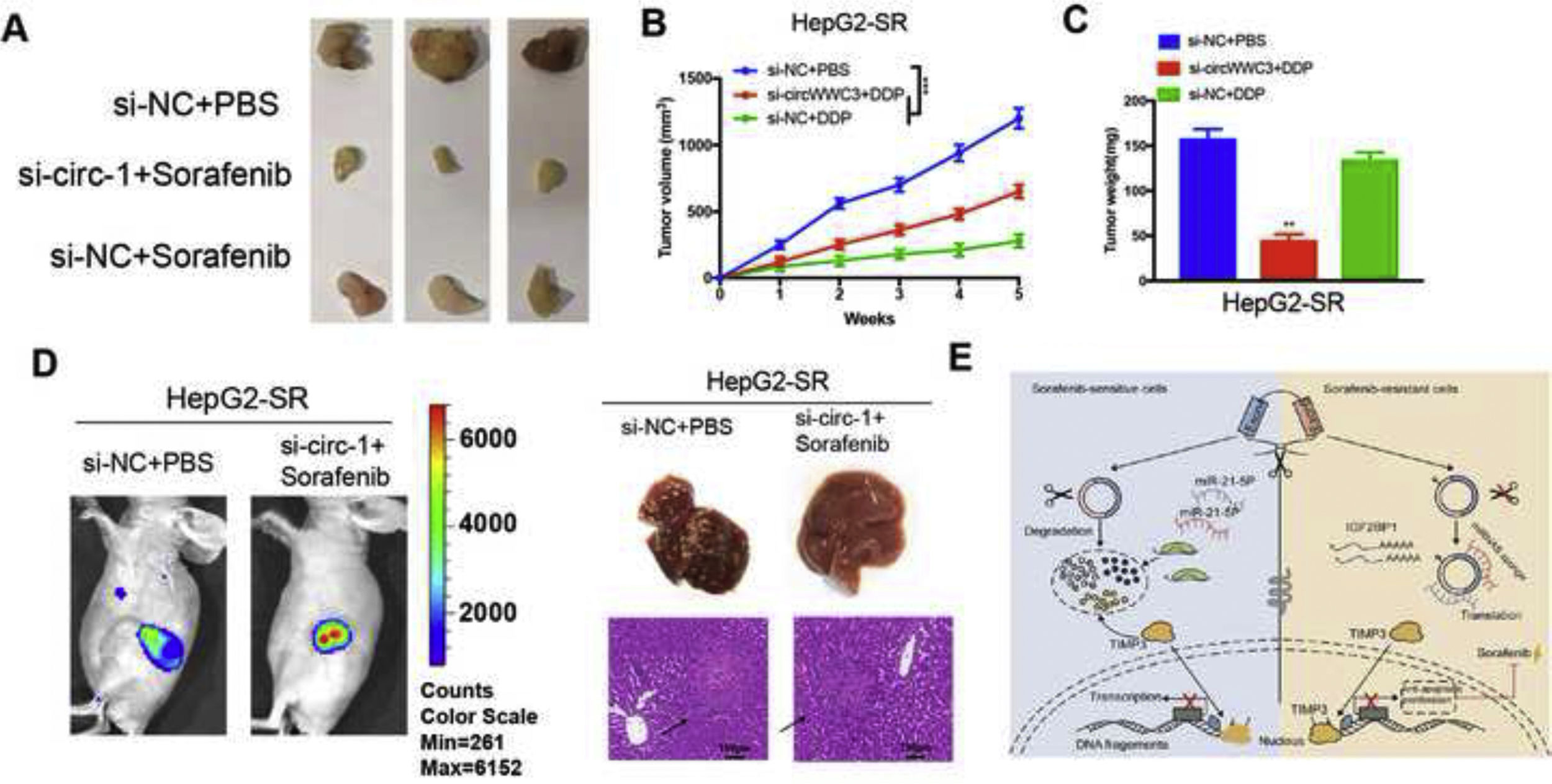
For in vivo investigation of clinical relevance of circRNA-001241, HepG2-SR cells with or without si-circRNA-001241 were subcutaneously injected to female BALB/c nude mice at their dorsal flanks and cell proliferation was allowed for the time period of 5 weeks after intraperitoneally injected sorafenib. Tumor xenograft data indicated that circRNA-001241 inhibition in HepG2-SR cells might suggestively reduce xenograft tumor growth and prepare cells to sorafenib treatment (Fig. 6A). The volumes and weights of tumors were pointedly (Fig. 6B and C). To investigate in vivo functions of circRNA-001241 in HCC cell metastasis, HepG2-SR cells either with or without si-circRNA-001241 cells were inoculated into mice’ tail vein. On 21st day, following sorafenib administration experimental mice were observed using live imager. The fluorescence signal intensity of si-circRNA-001241 group was significantly decreased compared with the control group (Fig. 6D). The results showed that the down-regulated expression of circRNA-001241 in sorafenib resistant cells preserved tumor metastatic levels in each liver metastasis model compared to the control group. Metastatic nodules in liver were significantly lower than those in control cells. Cumulatively, these results confirmed in vivo consequence of depriving circRNA-001241 on augmented sensitivity of sorafenib in HCC models, additionally assisting the clinical implication of circRNA-001241 silencing to advance sorafenib effectiveness in HCC patients. Overall, our results show that circRNA-001241 can regulate miR-21-5p/TIMP3signaling path, and inducing sorafenib resistance (Fig. 6E).
Circ-001241 promotes sorafenib resistance of HCC cells in vivo. (A) Murine xenograft tumors were treated with or without sorafenib (3mg/kg, three times a week) at the end of the experiment. (B) Growth curves of subcutaneous xenograft tumors. (C) Weights of tumors that developed in xenografts tumors. (D) Representative bioluminescent images of livers for each experimental group. Top, right, statistical analysis of bioluminescent tracking plots. Right representative liver and HE staining of liver metastatic lesions. (E) Schematic of the proposed model. circRNA-001241may act as a sponge of miR-21-5p to promote HCC sorafenib resistance by upregulate the level of TIMP3. Data are expressed as mean±SD. **P<0.01, ***P<0.001.
This study presented an integrative role of CircRNA-001241 in arbitrating HCC sorafenib resistance and regulatory phenomenon of miR-21-5p/TIMP3signaling path. Our data proposed that circRNA-001241 knockdown might enhance the sorafenib sensitivity in HCC cells. Our in vivo and in vitro experiments showed that circ-001241 retains the sensitivity of sorafenib to HCC cells and promotes cell proliferation. Direct contact between circRNA-001241, miR-21-5p and TIMP3 was confirmed by dual luciferase reporting system and RNA pull down. The circRNA-001241 inhibition might converse such properties and sensitize HCC cells to sorafenib. The results revealed that circ-001241 is of great importance in the development of sorafenib resistance in HCC cells.
In clinical practice, treatment options for HCC include surgery, percutaneous ablation, intra-arterial modalities and transplantation. In recent years, molecular targeted therapies and immunotherapy have also made great progress.15 However, none of these therapies has achieved the expected results, and the existing expert consensus recommendations lack evidence-based medical support. New effective therapies are urgently needed. Oral treatment with the multikinase inhibitor sorafenib is recommended globally as first-line therapy for advanced HCC16 and is supported by the results of several trials. However, acquired resistance usually begins to develop within 6 months of treatment, and sorafenib is effective in only 30% of patients with HCC.17 This high frequency of resistance greatly limits its clinical use, and resistance to sorafenib in HCC has not been characterized. Current clinical trials involving the combination of this multikinase inhibitor with other drugs may improve efficacy and reduce side effects. The key mechanism of action of immune checkpoint inhibitors (ICIs) is to block the immune failure or suppression pathway caused by chronic immune responses to tumor antigens, thereby reactivating the antitumor immune response.18 Among them, cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death receptor-1 (PD-1) and its ligand (PD-1) have been used to treat advanced HCC. For example, the PD-1 checkpoint inhibitor nivolumab has shown promising clinical results and is currently approved by the FDA for use in numerous malignancies, including HCC,19 and another ongoing clinical trial is combining nivolumab with the CTLA-4 negative modulator ipilimumab in HCC. These checkpoint inhibitors boost the patient's immune system and kill cancer cells, although PD-1, PD-L1 and CTLA-4 inhibitors have been shown to be effective in the treatment of patients with advanced HCC.20 In addition, ICIs can provide high objective response rates (ORR) when used as first-line treatment in advanced hepatocellular carcinoma, particularly pembrolizumab+lenvatinib (ORR 36%) or atezolizumab+bevacizumab (ORR 27.3%). These findings emphasize high therapeutic potential of ICI-based therapies in patients with advanced hepatocellular carcinoma.21 The combination of immunotherapy with other antitumor therapies such as surgery, chemotherapy, molecular targeted therapy and other combination therapeutic modalities is still the current standard of care.
CircRNAs, a category of ncRNAs, take an important part in the transcription and post-transcription pathways of gene expression.22 It has been reported that the comparative expression of circRNA is related to drug resistance in human samples. Take it as an example, circ RNA AKT3 up standardizes PIK3R1 to augment resistance of cisplatin in gastric cancer through suppression of miR-198.23 The circFOXM1 subsidizes to the sorafenib resistance in HCC by MECP2 regulation via miR-1324.24 The n6-methyladenosine adapted circRNA-SORE withstands sorafenib resistance in HCC by regulation of β-catenin signaling.25 Therefore, circRNAs may be a possible biomarker and therapeutic target for multiple drug resistance. Despite multiple clinical explanations, function of circRNA-001241 in mediating chemotherapeutic drug resistance in HCC patients is not clear yet.
MiRNAs are tinynon-coding RNAs (ncRNAs) with capability of gene expressions regulation.26 During tumor development and evolution, miRNAs may be involved in tumor genesis and the regulation of tumor microenvironment.27,28 Afterwards, it was confirmed that miR-21-5p interact straight with circ-001241 with luciferase reporter assay. It has been reported that miR-21-5p may inhibit cell proliferation, induce cell apoptosis and reduce cell migration by affecting many oncogenes in gastric cancer,29 colon cancer,30 breast cancer31 and non-small cell lung cancer cells.32 Our results showed that miR-21-5p was down-regulated in HCC tissues, which was consistent with previous reports.circRNA-001241 modulates HCC sorafenib resistance by sponging miR-21-5p.
Next, we discovered miR-21-5p targets and confirmed TIMP3 as an efficient target of miR-21-5p in HCC. TIMP3 is an important development-controlling gene which regulates cell differentiation and morphogenesis.33 Unusual TIMP3 genes expression has been found to be concerned in renal,34 colon,35 ovarian,36 mammary37 and pulmonary carcinomas.38 Though, the parts of TIMP3 in HCC sorafenib mediated resistance remain poorly described. Here we stated that, TIMP3 was recognized as an uninterrupted target of miR-21-5P and its expression was amplified in HCC. Further experiments regarding exploration of antagonistic roles of si-circRNA-001241 and si-TIMP3 in the modulation of HCC cells’ sorafenib resistance were performed. Consequently, circRNA-001241 expedites HCC sorafenib resistance by regulating the miR-21-5P/TIMP3 axis.
Finally, our study demonstrated the oncogenic role of circ-001241 in mediating sorafenib resistance in HCC, suggesting that the downregulation of circ-001241 in sorafenib resistant HCC cells may enhance its sensitivity to sorafenib treatment in vivo and in vitro. The potential factor for this phenomenon may be the regulation of the miR-21-5p/TIMP3 axis as a ceRNA. This study suggests that circ-001241 may be the trigger gene causing resistance to sorafenib in patients with hepatocellular carcinoma, and validates its resistance mechanism, providing a theoretical basis for a new mechanism of sorafenib resistance in patients with advanced hepatocellular carcinoma.
Conflict of interestThe authors declare no conflict of interest.
This project was supported by grants from Science and Technology Fund Project of Guizhou Health Commission [gzwjkj2020-1-100 (P. Liao) and gzwjkj2020-1-102 (Q. Yang)].