IgG4-related disease is a systemic disorder characterised by diffuse or tumoural inflammatory lesions. It can mimic pancreatic cancer, leading to increased rates of morbidity and mortality in patients as well as errors in diagnosis and treatment. The aim of this review is to take a differential diagnostic approach to these two entities using epidemiology, clinical and laboratory findings, imaging and histopathology.
La enfermedad relacionada con IgG4 es un trastorno sistémico caracterizado por lesiones inflamatorias difusas o tumorales. Puede simular cáncer pancreático, condicionando un aumento en la morbimortalidad de los pacientes y errores diagnósticos y terapéuticos. El objetivo de esta revisión es realizar una aproximación diagnóstica diferencial de estas dos entidades, desde el punto de vista epidemiológico, clínico, analítico, imagenológico e histopatológico.
IgG4-related disease (IgG4-RD) is a systemic disorder characterised by diffuse inflammatory or tumefactive lesions that exhibit a dense lymphoplasmacytic infiltrate with abundant IgG4-producing plasma cells, obliterative phlebitis and progression to storiform fibrosis.1 Presentation can be heterogeneous, synchronous or metachronous, with systemic or single-organ involvement.1–3 It is considered a great "mimicker" of malignant tumour diseases.1–5 The name IgG4-RD was designated in 2010 and accepted at the first international congress on this condition in Boston.1–5 The Boston congress led to the first consensus defining the distinctive pathological findings in 20124 and the first management and treatment guidelines published in 2015.5 The recent recognition of this disorder means morbidity and mortality rates can be high and diagnostic and therapeutic errors can be made when it is not considered among the differential diagnoses for cancerous lesions.1–5 Because of the common pancreatic involvement, IgG4 autoimmune pancreatitis (AIP) can mimic pancreatic cancer (PC).1,4
The main aim of this review is to develop a differential diagnosis for these two entities from a demographic, clinical, analytical, imaging and histopathological point of view.
Material and methodsWe carried out a non-systematic review of the literature in English and Spanish in Pubmed. We selected articles published in the last 10 years. Using the search strategy "Immunoglobulin G4 [MeSH] related disease" and "Pancreatic [MeSH] cancer", 5073 and 20,746 results were obtained respectively. The focus was on relevant and current information from high impact journals and/or expert authors in the different segments of these diseases.
Results: differentiation between AIP in IgG4-RD and PCEpidemiologyAs IgG4-RD was only recently recognised, its true prevalence and incidence are unknown and are probably underestimated.1–3 Some 80% of the data come from Asian countries, mainly Japan, where a prevalence of 2.6–10.2 cases per million population and an incidence of 336−1300 new cases per year have been reported.3 In the last few years, reports have increased worldwide, especially in Europe and North America.3
IgG4-RD is more common in males (male-to-female ratio 3:2).1–3 The mean age is 60.3
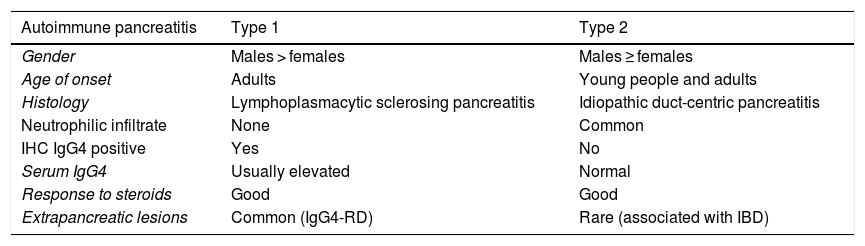
Pancreatic and submandibular gland involvement are the most commonly reported in the literature.1–4 Type 1 AIP is the pancreatic manifestation of IgG4-RD.1–3Table 1 shows the differences between AIP types 1 and 2. The prevalence of type 1 is no more than 11% of chronic pancreatitis cases.3 It has been reported in 98% of the international series of IgG4-RD with pancreatobiliary involvement and in up to 41% of systemic cases of the disease.3
Differences between type 1 and type 2 AIP.61
| Autoimmune pancreatitis | Type 1 | Type 2 |
|---|---|---|
| Gender | Males > females | Males ≥ females |
| Age of onset | Adults | Young people and adults |
| Histology | Lymphoplasmacytic sclerosing pancreatitis | Idiopathic duct-centric pancreatitis |
| Neutrophilic infiltrate | None | Common |
| IHC IgG4 positive | Yes | No |
| Serum IgG4 | Usually elevated | Normal |
| Response to steroids | Good | Good |
| Extrapancreatic lesions | Common (IgG4-RD) | Rare (associated with IBD) |
IBD: inflammatory bowel disease; IHC: immunohistochemical.
PC most often occurs in people in their 60 s and 70 s, with the mean age of onset being 71.6 In Spain, an incidence of 6.9 per 100,000 population was reported in 2015, including both males and females.7 Worldwide, PC has a male-to-female ratio of 1:1.6–10
Clinical manifestationsThe signs and symptoms of IgG4-RD at onset are heterogeneous and nonspecific.1–5 It frequently has a subacute or chronic course, and is often silent. However, it can be acutely brought to the fore because of a secondary complication.2 It can also be an incidental finding in an imaging test.1–5 The main signs and symptoms reported are described in Table 2. Asthenia is usually mild, weight loss does not rapidly progress to wasting syndrome, and hyporexia is rare in the absence of advanced disease.1–3 Acute pancreatitis is not common.1,3 Biliary involvement such as sclerosing cholangitis (IgG4-RD) occurs in 50%–90% of patients with pancreatic involvement,3 and can be complicated by infectious cholangitis and sepsis caused by cholestasis.1–3 Recent-onset diabetes has been reported in 40%−70%, with great variability between the different series, generally appearing when the disease has progressed to fibrosclerosis and significant replacement of the gland.1–5
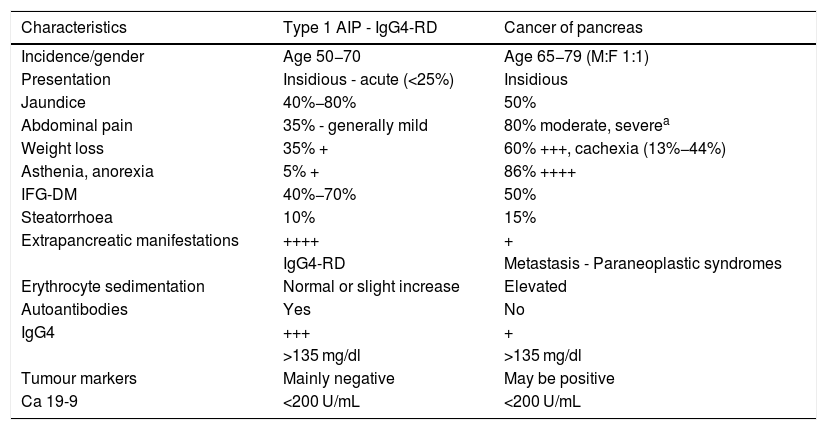
Clinical characteristics of type 1 autoimmune pancreatitis associated with IgG4-related disease and pancreatic cancer.
| Characteristics | Type 1 AIP - IgG4-RD | Cancer of pancreas |
|---|---|---|
| Incidence/gender | Age 50−70 | Age 65−79 (M:F 1:1) |
| Presentation | Insidious - acute (<25%) | Insidious |
| Jaundice | 40%−80% | 50% |
| Abdominal pain | 35% - generally mild | 80% moderate, severea |
| Weight loss | 35% + | 60% +++, cachexia (13%−44%) |
| Asthenia, anorexia | 5% + | 86% ++++ |
| IFG-DM | 40%−70% | 50% |
| Steatorrhoea | 10% | 15% |
| Extrapancreatic manifestations | ++++ | + |
| IgG4-RD | Metastasis - Paraneoplastic syndromes | |
| Erythrocyte sedimentation | Normal or slight increase | Elevated |
| Autoantibodies | Yes | No |
| IgG4 | +++ | + |
| >135 mg/dl | >135 mg/dl | |
| Tumour markers | Mainly negative | May be positive |
| Ca 19-9 | <200 U/mL | <200 U/mL |
DM: diabetes mellitus; IFG: impaired fasting glucose.
Isolated involvement of a single organ is very unusual.1–4 This makes the differential diagnosis with PC easier. It is important to ask about atopic symptoms such as bronchial asthma, rhinitis and chronic sinusitis, which can occur in up to 40%.1–3,11
Adenocarcinoma of the pancreas is the most common pancreatic cancer.6 It usually has an insidious onset, resulting in late diagnosis once the disease begins to cause significant symptoms due to local invasion or distant metastasis.6,9 The delay in diagnosis is greater when the cancer is located in both the body and the tail of the pancreas (20% of cases).9,10 The effects on the pancreas progress rapidly, leading to significant asthenia and weight loss.6,10 Abdominal pain is common and within a few months can become severe.6 Obstructive jaundice tends to occur earlier, and is more common with cancer of the head of pancreas.6,9 It may be associated with choluria, acholia and itching due to cholestasis.6,9,10
The Courvoisier-Terrier sign (painless palpable gallbladder) in a jaundiced patient without biliary colic, classically linked to malignant obstruction of the extrahepatic biliary tree (present in 13% in PC), is found in some forms of presentation of IgG4-RD.3,6,9 Other signs and symptoms include diarrhoea (44%), steatorrhoea (10%–25%) and vomiting (33%).6,9,10
Extrapancreatic involvement due to local invasion is common at diagnosis of PC.6 Infiltration of the vascular wall by the cancer can be similar to the periarteritis characteristic of IgG4-RD, lymphatic metastases can be confused with IgG4-related lymphadenitis, and carcinomatous infiltration of neighbouring organs can mimic retroperitoneal fibrosis.1,12
The rare paraneoplastic syndrome "panniculitis-arthritis-eosinophilia" could mimic IgG4-RD due to the combination of pancreatic lesion with eosinophilia.11
The similarity of the above signs and symptoms requires a broad and methodical differential diagnosis process through additional investigations, with priority given to the role of histopathology.5
Laboratory findingsAnalysisAmong patients with IgG4 AIP, 70%–80% have elevated transaminases and a cholestatic pattern.3 Unlike other inflammatory and autoimmune diseases, erythrocyte sedimentation and C-reactive protein are usually normal.1–5 Other nonspecific findings are: variable increase in amylase and lipase (generally mild-to-moderate), anaemia of chronic diseases, variable eosinophilia, and increase in immunoglobulin E in 20%–40% of cases.1–5,11 Complement levels are generally normal when there is pancreatic involvement, but hypocomplementaemia is seen in up to 25% of patients with submaxillary, pulmonary and aortic involvement, and is considered a marker of activity in renal involvement.1,3 More than 60% of cases have polyclonal hypergammaglobulinaemia.1–5
In PC there may be mild anaemia or reactive thrombocytosis, increased transaminases and cholestasis enzymes, and mildly elevated amylase and lipase (35%).9 Only 5% of patients develop acute pancreatitis.6,7
Serum IgG4IgG4 is the least abundant immunoglobulin G isotype (less than 5% in healthy patients).1–3 Its structure consists of two heavy and two light chains. Unlike other subtypes, the disulfide bridges that join the two heavy chains are unstable.1 This allows for random separation and recombination with fragments of other IgG4 molecules.1–3 The formation of new bivalent molecules allows it to act like antibodies with two different antigen-binding specificities, but, unlike the other IgG subtypes (IgG1, IgG2, and IgG3), IgG4 lacks capacity for antigen exchange or immune complex formation.1–5 Values below 140 mg/dl are considered normal.13,14
A 2007 study compared total IgG and IgG4 levels in 45 patients with AIP and 135 with PC.13 The authors found elevated levels of total IgG in 42% of AIP cases versus 5% in PC, and IgG4 ≥ 140 mg/dl in 76% of patients with AIP (mean 550 ± 98 mg/dl) versus 10% in PC (mean 70 ± 9 mg/dl, OR: 37).13 When raising the cut-off point 280 mg/dl, only 1% of patients with PC had elevated levels, compared to 53% in AIP.27 A prospective study also concluded that levels ≥280 mg/dl showed greater specificity (Sp) to distinguish IgG4-RD from other inflammatory disorders and cancers (Sp 96.2%, negative predictive value [NPV] 97.7%, and sensitivity [Se] 56.9%).14 A recent meta-analysis concluded that a serum IgG4 cut-off of 135–144 mg/dl has an Se of 87% and an Sp of 83% for the diagnosis of IgG4-RD.15 When a cut-off value of 280 mg/dl was used, the pooled Se decreased to 63%, but Sp was 95%.15 The above meta-analysis included a modest number of studies, but showed significant heterogeneity regarding the values to be considered positive. Therefore, although this marker has value in the appropriate clinical context, its diagnostic performance is lacking.13,15 Elevated concentrations of serum IgG4 have also been found in other diseases mediated by this molecule (membranous glomerulonephritis, thrombocytopenic thrombotic purpura, pemphigus foliaceus) and in lymphoproliferative processes, chronic respiratory diseases, primary sclerosing cholangitis, cholangiocarcinoma, atopic dermatitis, parasitic diseases, etc.1–3,13,15 However, in these processes, the values are usually below 280 mg/dl.13,15
We should stress that 20%–30% of patients with IgG4-RD have normal serum levels of IgG4.1,14,15 Some authors believe that this may be due to the involvement of few organs or advanced fibrotic disease, the variability of criteria applied in the different series, ethnic and geographic differences, or the immunological method used to detect this IgG,1–3,14–16 Another possible reason for false negatives is the prozone effect.17–19 Nephelometry tests for IgG4 are prone to error in the presence of excess antigen, and may underestimate the concentration because flocculation does not occur.17,18 Proper dilution of the serum sample during the assay process would avoid this effect.17
For all of the above reasons, this method lacks specific weight alone to differentiate ER-IgG4-RD and PC.
Identification of circulating plasmablastsThe pathophysiology of IgG4-RD is still not fully understood.1–3 It has been suggested that in individuals who are genetically predisposed, an environmental trigger or a microorganism could cause altered self-antigen presentation, defects in innate immunity and loss of immune tolerance.1 This would produce an imbalance between T helper (Th) 1 and 2 lymphocytes with an increase in the response of the Th2 cells.1,2 The activated Th2 cells secrete interleukin (IL)-5, responsible for peripheral and tissue eosinophilia, and IL-13, which stimulates the proliferation of B cells and plasma cells which produce IgG and IgE.1,2 This process induces the response of IL-10-producing conventional and memory regulatory T cells (Treg, CD25+, FoxP3+).3,20,21 IL-10 is responsible for the change to the IgG4 subclass in the B cells and for the production of transforming growth factor®, which stimulates the participation of fibroblasts.1,3 All this leads to infiltration of plasma cells, eosinophils and fibroblasts, causing tissue damage.1–3 At the same time, follicular Th lymphocytes induce the development of germinal centres in the lymph nodes and the generation of IgG4-secreting plasmablasts (PB) and long-lived plasma cells.21,22 Based on this knowledge, advances have been made in understanding the role of PB in the disease.21
The concentration of circulating PB can be analysed by flow cytometry, detecting different markers present on their surface.21,22 This determination has shown utility as a biomarker, as PB increase when the disease is active (even in the presence of normal serum IgG4), they decrease dramatically when patients respond to rituximab, and they become elevated in relapses.21,22 However, the availability of this technique limits its use in daily practice.
PB are not usually found in healthy subjects, except for low and transient levels after vaccination or some infectious processes.21 Nonetheless, they can be found in other inflammatory/autoimmune diseases and in haematological disorders.22
The PB concentration has not yet been analysed in selected populations with PC.21
Tumour markersNumerous studies have examined the utility of tumour markers for the screening and diagnosis of PC.9,10 The most studied is the serum cancer antigen 19-9 (CA 19-9).9,23 In 10% of people (with a negative Lewis genotype) it is not a useful marker.9 In asymptomatic patients, Se and Sp show great variability (70%–92% and 68%–92%, respectively).23 Due to its low Se, expert guidelines do not recommend it as a screening method for PC, but it is useful for patient follow-up after surgery.9
The Se is very low in small tumours.9,23,24 Among other conditions, it can be elevated in acute and chronic pancreatitis, liver cirrhosis, cholangitis, cholelithiasis, neuroendocrine tumours of the pancreas and biliary or hepatocellular cancer.23,25 Even in symptomatic patients, the cut-off value of 37 IU/mL would only enable differentiation of PC from benign disease with an Se of 77% and Sp of 87%, and a positive predictive value [PPV] of 72%.13 CA 19-9 is elevated in 27% of patients with AIP.13 A higher cut-off point (100 IU/mL) increases the Sp for PC (71% in PC versus 9% in AIP).13 However, there is no CA 19-9 level (even >10,000 IU/mL) which is seen exclusively in patients with PC.13,23 These limitations therefore need to be considered when requesting this marker to differentiate between the two diseases.
AutoantibodiesThere are no specific or sufficiently sensitive antibodies (Ab) associated with IgG4-RD.1–3 There are reports of anti-lactoferrin Ab, anti-carbonic anhydrase II, anti-plasminogen-binding protein Ab and pancreatic secretory trypsin inhibitor being associated with AIP, but the value of this association in clinical practice is uncertain.26,27
IgG4 Ab can bind to the Fc region of IgG producing a rheumatoid factor (with an unknown pathogenic role) present in up to 20% of patients.1–3 Antinuclear Ab (generally low titres) can also be found and anti-Ro/SSA and anti-La/SSB have been reported in patients with IgG4-RD.1,2 However, the presence of these Ab makes it necessary to rule out Sjögren's syndrome.1,3 Antimicrosomal and anti-smooth muscle Ab can be seen in this disease, but whether or not this is an association with autoimmune liver disease has yet to be determined.27
The presence of antineutrophil cytoplasmic Ab (ANCA) makes it necessary to rule out necrotising vasculitis.28 The finding of anti-DNA, anti-Sm, anti-RNP and cryoglobulins should alert to the possibility of another systemic disease in the appropriate clinical context.28
The presence of Ab in IgG4-RD currently lacks diagnostic utility and does not help differentiate this disease from PC.20,23
Diagnostic imagingIgG4 AIP can present three patterns: diffuse, focal or multifocal.1–3,28 The diffuse, known as "sausage-shaped" pancreas, is the most common.1–3 It is characterised by a homogeneous increase in gland size and loss of lobulations.1–5 It is frequently associated with IgG4-related sclerosing cholangitis (IgG4-SC).1,27–29 One of the keys to differentiating this disease from PC is to find alterations in other organs.1–5,12,29 In the kidneys, for example, unilateral or bilateral cortical nodular lesions, bilateral diffuse cortical enlargement and/or involvement of the renal pelvis can be found in IgG4-RD.1–3,29,30 In the retroperitoneum, there is often swelling surrounding the abdominal aorta (periaortitis) and its branches and enveloping the ureters and the renal pelvis, causing hydronephrosis.1–3,31 Sclerosing mesenteritis can also be confused radiologically with lymphoma, fibromatosis and neuroendocrine tumours.1,2,30
While PC infiltrates vessels and organs, IgG4-RD surrounds and encompasses them.31,32 However, there can be uncertainty about the difference when both diseases are in advanced stages.31
Conventional and contrast-enhanced abdominal ultrasound (US)Conventional US continues to be the technique of choice in the initial study of disease of the pancreas.30,31 Presence of a hypoechoic mass at the head and dilation of the pancreatic and biliary ducts are suggestive of PC.32,33 In body and tail involvement, visualisation is difficult due to the lack of bile duct dilation and the presence of gas in the stomach and transverse colon.33 The Se and Sp of conventional US also depend on the experience of the operator, the stage of the disease and the patient's build.31,33 For these reasons, its diagnostic accuracy in PC is questionable and the Se ranges from 50% to 90%.30–33 Contrast improves visualisation, in some cases enabling focal tumours suggestive of malignancy to be differentiated from fibroinflammatory lesions.31–33
IgG4 AIP exhibits a diffuse increase in gland size, reduced echogenicity, and a decrease in the pancreatic duct lumen due to compression of the affected parenchyma.31 Contrast administration reveals moderate to intense enhancement (depending on the degree of fibrosis and thinning of the pancreatic vessels due to lymphocytic infiltration) in the early phase, with a slow and progressive washout.1,31 Contrast is very useful in differentiating the focal and multifocal pattern of AIP from PC.31
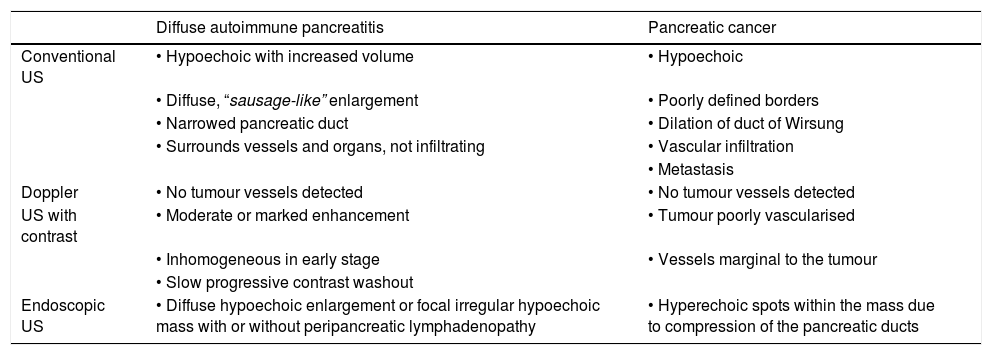
The main differences on ultrasound between these two disorders are shown in Table 3.
Differential ultrasound features between type 1 autoimmune pancreatitis and pancreatic cancer.
| Diffuse autoimmune pancreatitis | Pancreatic cancer | |
|---|---|---|
| Conventional US | • Hypoechoic with increased volume | • Hypoechoic |
| • Diffuse, “sausage-like” enlargement | • Poorly defined borders | |
| • Narrowed pancreatic duct | • Dilation of duct of Wirsung | |
| • Surrounds vessels and organs, not infiltrating | • Vascular infiltration | |
| • Metastasis | ||
| Doppler | • No tumour vessels detected | • No tumour vessels detected |
| US with contrast | • Moderate or marked enhancement | • Tumour poorly vascularised |
| • Inhomogeneous in early stage | • Vessels marginal to the tumour | |
| • Slow progressive contrast washout | ||
| Endoscopic US | • Diffuse hypoechoic enlargement or focal irregular hypoechoic mass with or without peripancreatic lymphadenopathy | • Hyperechoic spots within the mass due to compression of the pancreatic ducts |
US: ultrasound.
Abdominal US is an acceptable first imaging method but does not provide diagnostic certainty.31–33
Conventional and endoscopic ultrasound elastographyNew equipment, second-generation techniques, the endoscopic method and computer programs have improved the results of this study, reducing interpretation biases.34,35 However, there have been few studies in this field and with a limited number of cases.35–40 Elastography studies have shown inconsistent results regarding the hardness of pancreatic lesions specifically associated with AIP.41 One study reported an Se of 100% and Sp of 67% for the detection of pancreatic tumours.36
Another study with endoscopic ultrasound elastography included 130 consecutive patients with solid pancreatic lesions and 20 healthy controls and defined four different elastographic patterns: homogeneous green (healthy controls); heterogeneous, predominantly green (mainly inflammatory lesions and absent in PC); heterogeneous, predominantly blue (adenocarcinomas and other neoplasms); and a homogeneous blue pattern (only in neuroendocrine tumours).39 The authors concluded that a predominantly green, homogeneous or heterogeneous pattern excludes malignancy with high precision.37 The Se, Sp, PPV, NPV and overall precision of endoscopic elastography for the diagnosis of malignancy were 100%, 85.5%, 90.7%, 100% and 94% respectively.37 However, other previous studies with fewer patients obtained a similar Se but a clearly lower Sp (60%–70%).38,39
A meta-analysis that included seven studies and 752 patients revealed an overall Se of 97% and Sp of 76%.40
Therefore, this technique can complement the clinical assessment and other imaging studies in centres which have the suitable equipment and personnel, mainly in terms of allowing malignancy to be ruled out and facilitating biopsy.35,42
Endoscopic ultrasoundEndoscopic ultrasound (EUS) provides greater resolution of the pancreatic parenchyma than conventional US.43 It has shown greater diagnostic performance than positron emission tomography (PET-CT), computed tomography (CT) and abdominal US for recognising pancreatic tumours in early stages (Se 99% vs 55% for CT).43,44 It has the disadvantage of being an invasive technique, although the rate of complications is low (1%–3%).44 In AIP 1, EUS makes it possible to visualise the diffuse or focal hypoechoic enlargement of the gland and the peripancreatic lymphadenopathy.41 In the focal pattern, unlike in PC, hyperechoic spots can usually be seen within the mass caused by lymphocytic infiltration and compression of the ducts.1–3,40,41 Apart from these subtle changes, which are not always present, there are no specific characteristics to differentiate AIP from PC.
If contrast is added, AIP lesions may be homogeneously hypervascularised, whereas PC is mainly hypovascular (Table 3).41
The biggest advantage of EUS is that it can be used to guide the needle biopsy.43–45
Computed tomography with intravenous contrastThis enables visualisation of the pancreas and the extent of the inflammatory or cancerous process.32,42
In diffuse AIP, the "sausage-shaped" pancreas is seen with a "halo sign" or "false capsule", hypodensity which appears in late phase surrounding the gland and allows it to be differentiated from lymphoma.1–3 The duct is often irregular with stricturing from surrounding inflammation.1–3,33 The focal pattern is usually hypodense in early phase and isodense in late phase, a pattern of behaviour similar to PC.1,32,33 In this case, extrapancreatic findings are more valuable.1–3
CT is very useful when PC is suspected due to its wide availability and experience in the interpretation of results. It has Se > 90% and Sp of 99%.32,33,45,46 The biggest limitation is the lower Se for early lesions and tumours smaller than 2 cm.30 These values improve with triphasic helical computed tomography.47 The protocol for pancreas includes an arterial phase with maximum enhancement of the aorta at 30 s, a "pancreatic" phase with greater contrast between tumour and parenchyma at 40 s, and a portal venous phase with maximum enhancement of the liver between 60 and 70 s.47 The arterial and venous phases contribute to staging by assessing vascular involvement, and the portal venous phase enables identification of liver metastases.47 PC is seen as a poorly defined mass, hypodense with respect to the rest of the pancreas, with a heterogeneous pattern and spiculated margins.30,32,46–48 However, small, low-grade tumours may appear isodense relative to the normal parenchyma and require secondary signs for recognition (focal ductal disappearance, distal ductal dilation, distal parenchymal atrophy, pancreatic contour deformity).46–48 In late phase, there is a heterogeneous increase in intensity and peripheral enhancement, and peripancreatic strands can be seen extending into the surrounding adipose tissue.32,33,46–48 Unlike in AIP, the duct usually has smooth contours, with focal stricturing or amputation at the tumour site and pre-obstruction dilation.30,31,46–48 Simultaneous dilation of the pancreatic and bile ducts (double-duct sign) is present in 70% of cases of PC located in the head of pancreas, but it is not pathognomonic and can be seen in chronic pancreatitis.31 Moreover, there is often disorganised infiltration of the vascular walls and neighbouring organs which may be indistinguishable from AIP with extensive inflammation of the peripancreatic tissues.45,46
Magnetic resonance cholangiopancreatographyMagnetic resonance cholangiopancreatography (MRCP) is a useful technique to demonstrate anatomical alterations of the biliary system and pancreatic ducts.
The findings are similar to CT but can provide differential information on biliary involvement in patients with cholecystitis and/or IgG4-SC.1–3,28 A retrospective study that included 162 patients (47 with IgG4-SC, 73 with primary sclerosing cholangitis [PSC], and 42 with autoimmune liver disease) analysed MRCP findings in bile ducts, liver, pancreas and other organs.49 The authors showed that IgG4-SC was significantly associated with contiguous thickening of the intra- and extrahepatic bile ducts, abnormalities of the pancreatic parenchyma, kidney abnormalities and thickening of the gallbladder wall.49 The thickness of the common bile duct wall was significantly greater in IgG4-SC (mean 3 mm) compared to PSC (1.89 mm) and the group with autoimmune liver disease (1.80 mm).49 However, they found no statistically significant differences between the three groups regarding the location or length of the extrahepatic bile duct strictures, abdominal lymphadenopathy or retroperitoneal fibrosis.49
In a study that compared MRCP with endoscopic retrograde cholangiopancreatography (ERCP) for detecting PC, the Se for MRCP was 84% and the Sp 97%, comparable to those for ERCP, which were 70% and 94% respectively.50 Head of pancreas tumours can also cause common bile duct obstruction.9,10 If PC is suspected, the examination requires the addition of T1-weighted gradient-echo and T2-weighted images with administration of contrast.9,27,30,50 PC is characteristically hypointense on T1-weighted images compared to normal parenchyma.30,50 Diffuse, segmental or focal broadening of the pancreas may be seen, with delayed enhancement or hypoattenuation.50
Endoscopic retrograde cholangiopancreatographyA number of studies have shown that ERCP, MRCP and EUS allow adequate visualisation of biliary strictures.34,40,45 ERCP associated with EUS yielded better results in the differential diagnosis between benign and malignant disease, but EUS is less widely available.34,40,45
The findings for AIP in ERCP are: stricture affecting more than a third of the length of the pancreatic duct; absence of dilation proximal to the stricture site; multiple areas of stricturing; and secondary branches emerging from the narrowed segment.28 The bile duct is affected simultaneously in 20%–80% of patients.28,30 Four patterns of biliary involvement have been described in IgG4-SC.28 Type 1 involves the distal area of the common bile duct, and differential diagnoses should include PC, distal cholangiocarcinoma, and involvement secondary to chronic pancreatitis.28 Some experts have reported that, unlike cholangiocarcinoma or PC, IgG4-SC does not usually involve pre-stricture dilation.28,31
F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET/CT)This technique enables the assessment of extrapancreatic involvement.51 In a prospective study assessing 35 patients with IgG4-RD, PET/CT identified multiorgan involvement in 97%, and in 71% it detected involvement of at least one organ which had not been detected by physical examination, US or CT.52 According to the authors there is a specific image and pattern in IgG4-RD, including elevated 18F-FDG uptake in the pancreas and salivary glands, patchy lesions in the retroperitoneal region and vascular walls, and multi-organ involvement which cannot be interpreted as metastasis.52 In seven cases, the new PET/CT findings made it possible to re-select the biopsy site for more accessible lesions, such as peripheral lymph nodes and submandibular glands.52 In addition, they showed that after two to four weeks of treatment with 40 mg of prednisone per day, 72% showed complete remission and the remainder a decrease in 18F-FDG uptake of >80%.52 Another study which examined the utility of PET/CT in 26 patients with IgG4-RD showed that all patients had two or more affected organs, with a mean standardised uptake value (SUV) of 4.14 (range 0.30–8.78).53 These authors also reported that 11 patients had been wrongly diagnosed with submandibular tumours, PC, pancreatitis, pulmonary interstitial fibrosis, retroperitoneal fibrosis or systemic vasculitis before the PET/CT.53 A recent case-control study aimed at assessing the utility of PET/CT for diagnosing AIP and ruling out PC included 53 patients with suspected AIP who had a PET/CT before treatment, and compared their scans with the PET/CT of 61 patients with PC.54 The researchers found significant differences between the two groups in pancreatic tumour uptake morphology, SUV, texture characteristics of the primary tumour, and in the number and location of extrapancreatic foci.54 Using a prediction model, the area under the receiver-operating-characteristic curve was 0.95 (P < .0001), with Se, Sp, PPV and NPV of 90.6%, 84.0%, 87.9% and 87.5% respectively in the differentiation between AIP and PC.54 Therefore, the authors concluded that PET/CT offers a high sensitivity to differentiate AIP from PC and that systemic inflammatory foci help to confirm the diagnosis of AIP in patients who have not started corticosteroid therapy.54
The aforementioned studies conclude that it is a useful tool for the differential diagnosis of IgG4-RD, to assess systemic involvement, guide the biopsy and monitor the response to treatment.52–54
The role of PET/CT in PC remains the subject of debate. Its Se and Sp have been reported in the ranges of 73%–94% and 60%–89% respectively.6,9,55 Despite its greater use in malignant diseases, we do not know whether it provides more information than that obtained with a triphasic helical CT in a patient with suspected PC.6,9 Some studies agree and others disagree about whether PET/CT has greater sensitivity to diagnose small metastatic lesions, with its resolution limit close to 8 mm, similar to that of CT.55 Another false negative includes hyperglycaemia.51–56
A recently published British multicentre study assessed the utility of adding PET/CT to CT in 550 patients with suspected PC and, based on the results, the authors decided in favour of the more extensive use of this technique.55 The Se, Sp, PPV and NPV when performing both methods slightly exceeded those of CT alone.55 The incremental likelihood ratios demonstrated that PET/CT significantly improved diagnostic accuracy in all scenarios (P < .0002), correctly changed PC staging in 56 cases (P = .001) and influenced treatment in 45% of the patients.55 It also made it possible to cancel resection in 20% of patients who were to undergo surgery.55 However, the authors accepted that the benefits of PET/CT were limited in patients with chronic pancreatitis or other pancreatic tumours.55 With the results obtained, PET/CT was considered only to be more cost-effective for the subgroup of patients with suspected PC which had erroneously been considered as resectable.55,56
In conclusion, despite some promising results, this tool is expensive and of limited availability. Its real applicability in routine clinical practice and its ability to measure activity and aid therapeutic decisions requires a great deal of further study.5,51 The current IgG4-RD consensus guidelines therefore warn that as the use of PET/CT cannot be standardised or generalised, its utility needs to be assessed on a case-by-case basis by the treating physicians.5,51
Therapeutic responseNumerous studies have shown that AIP responds to prednisone treatment with almost complete recovery, except in very advanced cases where the response is partial.1–3,5 In patients with strong suspicion of AIP, therapeutic response to corticosteroids is considered a HISORt diagnostic criterion (histology, images, serology, other organs involved, response to treatment).56 Generally there is radiological remission after 2 weeks of optimal therapy (prednisone 40 mg/day).56 In cases with strong suspicion of inflammatory disease, monitoring with imaging at 8–12 weeks is recommended to check for complete response.11,56 However, when cancer is suspected, a lack of therapeutic improvement at 2 weeks makes it necessary to formally rule out malignancy.5 Patients with PC may experience transient relief of symptoms during the steroid trial due to a reduction of the inflammation around the tumour.56 The response should therefore be assessed with diagnostic imaging methods looking for resolution of the pancreatic mass.1–3,56
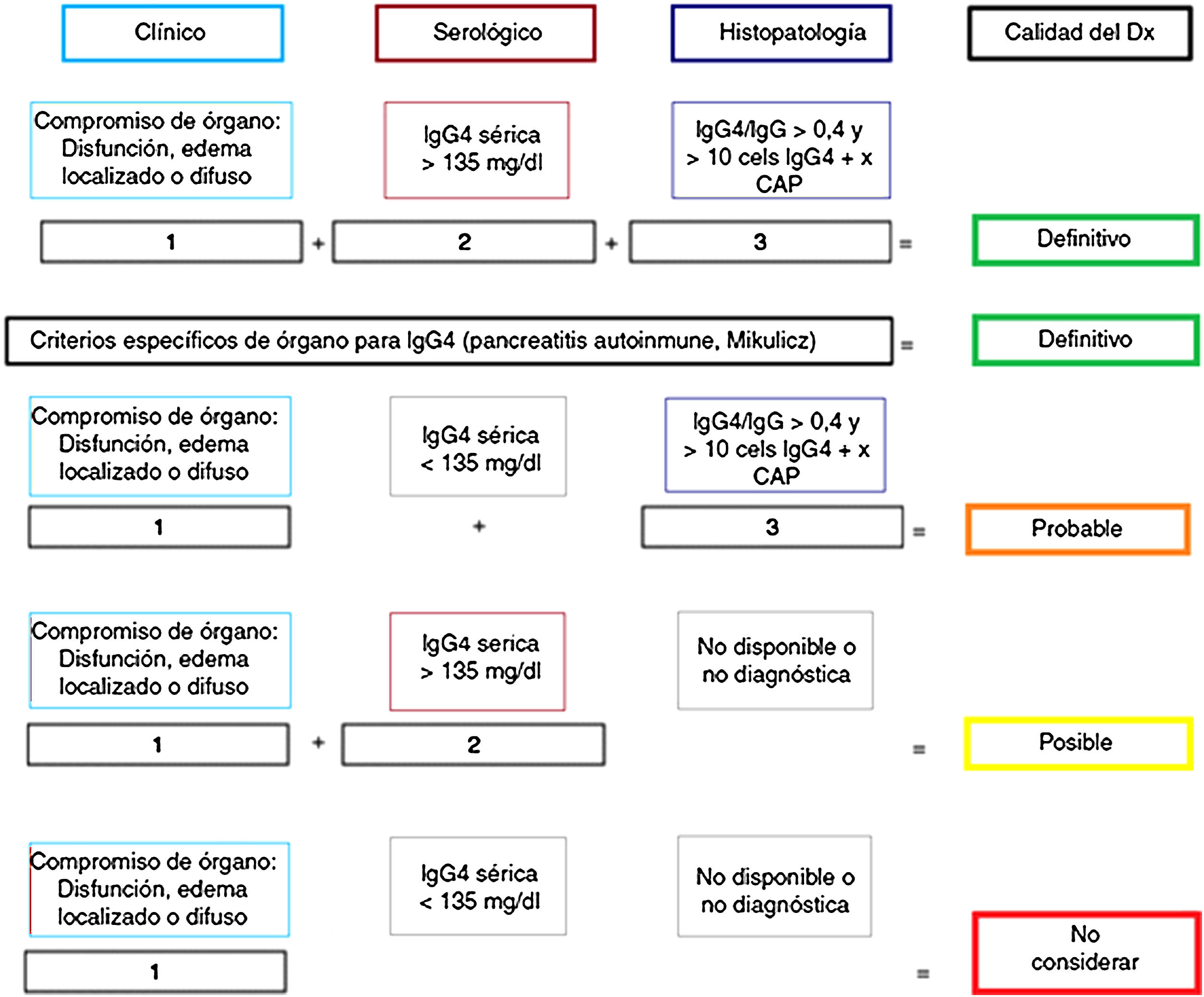
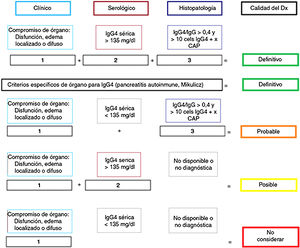
PathologyImportance of the histology studyAlthough “diagnostic” criteria have been proposed for IgG4-RD57,58 (Fig. 1), as in other autoimmune and inflammatory diseases, their greatest utility is for classifying patients for scientific trials and they are no substitute for clinical judgement.5 Clinical, analytical and radiological assessments are often insufficient to distinguish between IgG4-RD and PC.1,25,58 For that reason, the IgG4-RD international consensus guidelines strongly recommend (94% expert agreement) performing histological confirmation whenever possible.5,58
Certainty of the diagnosis of IgG4-RD according to Umehara's diagnostic criteria.
Adapted from Ardila-Suarez et al.58
However, it has to be acknowledged that obtaining biopsies can be difficult at times, and that they lack the intrinsic value of certainty if not correlated with the other tests.5,29 The effectiveness of the procedure depends on the quality of the sample, the selected site, the route by which it is obtained, the processing and the interpretation of the results.4,29,58,59 The NPV of biopsy in IgG4-RD is lower than in PC.6,58
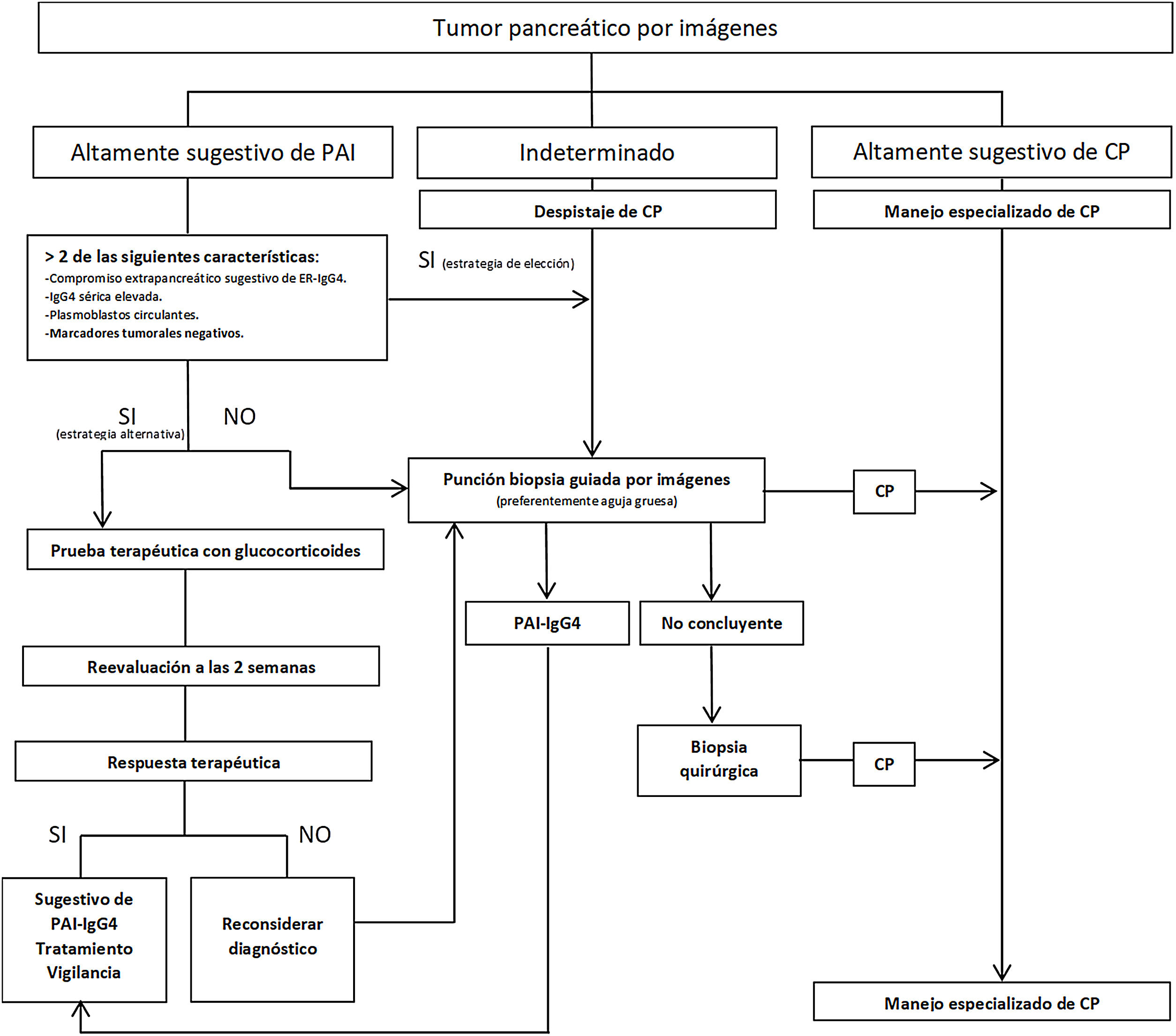
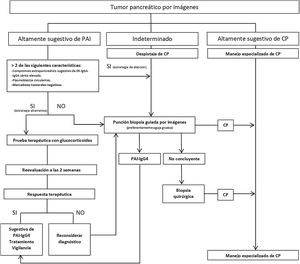
Strategies have been proposed to distinguish IgG4 AIP from PC (Fig. 2), stratifying patients based on radiological findings into three groups: 1) imaging highly suggestive of AIP; 2) indeterminate (focal, multifocal or advanced diffuse lesions with peripancreatic invasion); and 3) imaging highly suggestive of PC.59 Although histological confirmation is desirable in all scenarios, in group 1 patients who have risks and absolute contraindications, inaccessibility, who refuse to have a biopsy, and who also have involvement of other organs suggestive of IgG4-RD and/or elevated serum IgG4 levels, and/or elevated PB and negative tumour markers (taking into account the limitations of these methods), steroid therapy (prednisone 40 mg/day) could be started and therapeutic response evaluated with CT or MRCP at 2 weeks. If there is a response, the diagnosis of AIP can be considered while monitoring the patient closely. However, despite being able to diagnose AIP, a definitive diagnosis of IgG4-RD cannot be established unless it is confirmed histologically in pancreas or other tissue. In the other two groups, malignancy must be ruled out with histology and immunohistochemistry tests.1–6,29,57,59
Obtaining the sampleThe most suggestive morphological characteristics of IgG4-RD in pancreatic tissue require that the sample preserve the glandular architecture.4,29,58 For this reason, performing fine needle biopsies (FNA) to diagnose IgG4-RD is not advised.5,58
The strategy of obtaining a pretreatment biopsy (prior to surgery or oncological therapy) is increasingly common when PC is suspected, especially in patients with an advanced lesion or who will need neoadjuvant treatment.6,9 In this case, US- or CT-guided FNA has an Se of 80%–90% and Sp close to 100%.59 It has the advantages of being a widely used technique (more experienced operators), being cheaper and not requiring sedation.59,60
The need for a biopsy in a patient with an initially resectable lesion continues to be the subject of debate, as if it is negative, it does not remove the need for surgery in a patient with strongly suspected PC and there would be a potential risk of dissemination to the peritoneum.6,9 It has been suggested that FNA guided by transduodenal EUS has an Se of 90% and Sp of 96%, and by not going through the peritoneum, would reduce the risk of dissemination.60 However, this technique mainly obtains isolated cells, making it difficult to diagnose well-differentiated tumours or those with marked desmoplasia.60 Therefore, whenever anatomically possible, in centres with availability and experience, the preference is for endoscopic ultrasound or percutaneous core needle biopsy (Tru-Cut), which has a complication rate very similar to that of fine needle biopsy (around 2%) and the advantage of requiring fewer punctures.6,9,59,60
In AIP, FNA has shown an Se of 36% and Sp of 33%, compared to core needle biopsy, which in some studies reached an Se and Sp of 100% when taken from a highly suggestive lesion and guided by EUS.5,44,45,57,59 A Tru-Cut biopsy with a 19 G needle can also be guided by CT or conventional US.5,44,45
Difficulties also arise when the samples show nonspecific chronic pancreatitis, as these changes may correspond to an area in the vicinity of a PC or AIP.1–3,5,59 In such cases, or in situations of uncertainty (atypical clinical progress, lack of involvement of other organs, absence of peripheral halo in the mass, nonspecific ductal morphology, normal levels of IgG4, etc.) it may be necessary to repeat the needle biopsy or obtain surgical biopsies through exploratory video laparoscopy, avoiding unnecessary pancreaticoduodenectomy if the evidence does not point strongly to PC.1,5,12,29,56,59 Intraoperative frozen section biopsies can be difficult to interpret due to the intense desmoplastic reaction that can often be seen in IgG4-RD, which can confuse the diagnosis with PC.1,4,12,29,57,59
Histological and immunohistochemical findingsThe histological findings of IgG4 AIP are: dense lymphoplasmacytic infiltrate (Se 100%, Sp 17%) with plasma cells positive for IgG4, in a context of fibrosis with a storiform pattern (Se 31%, Sp 100%) and obliterative phlebitis (Se 54%, Sp 100%).1–5,57,58 Other possible findings are: non-storiform fibrosis (Se 91%, Sp 82%), non-obliterating phlebitis, and mild-to-moderate eosinophil infiltration (Se 43%, Sp 100%).4,57,58 More than 10 IgG4+ plasma cells per high-power field (HPF) are required for needle-biopsy samples, and > 50 IgG4+ cells per HPF for surgical samples.4,57,58 However, it is important to bear in mind that the phase of progression of the disease may affect the results.57 Pancreatic disease with significant retroperitoneal fibrosis is often diagnosed late and may show paucicellular fibrosis.57 Consequently, some researchers believe that an IgG4:IgG ratio in plasma cells of >40% is highly suggestive when supported by clinical and imaging features.4,29,57,58
The simple presence of IgG4+ cells in other tissues without compatible histological findings is also not sufficient for diagnosis, as this can be found in other disorders: diverticulitis, anti-neutrophil cytoplasmic antibody [ANCA] vasculitis, multicentric Castleman disease.4,58 PC can also be infiltrated by IgG4+ plasma cells to varying degrees, and this phenomenon can also be observed in other cancers and in regional lymph nodes.4,29,57 In these cases, the infiltration is usually patchy and is not associated with other histological characteristics of IgG4-RD.1,4 The discussion continues about whether or not the cases reported as synchronous PC and IgG4-RD represent a true association, the findings of IgG4+ cells in the areas adjacent to the PC are an epiphenomenon, or the association is just a coincidence.1–5,29,58 More tissue should therefore be obtained in uncertain cases. The diagnosis of IgG4-RD can be made if at least two of the three basic features of IgG4-RD are present, along with a significant increase in IgG4+ or IgG4/IgG cells of >40%.14,29 If these conditions are not met, the diagnosis is only probable and additional evidence is needed, such as elevated serum IgG4, PB, radiological findings and/or systemic involvement.29
In PC, cancer cells tend to form glands and infiltrate the dense stromal fibrosis (ductal adenocarcinomas), for which they are known as scirrhous or desmoplastic tumours.6,9 For FNA biopsies in cases with extensive fibrosis, many samples are often required to find atypical cells.9
Clear cell, adenosquamous, undifferentiated or anaplastic, and non-cystic mucinous carcinoma are some of the subtypes of ductal PC.6,9 Other variants which develop as solid masses are acinic cell carcinoma and solid pseudopapillary tumours (uncommon and with a better prognosis).6,9 They have a tendency to invade neighbouring tissues, blood vessels and nerve structures.9
ConclusionsMaking the differential diagnosis between AIP in IgG4-RD and PC requires a multidisciplinary approach which must include discussion of clinical, serological, radiological and histological findings. These processes should be confirmed histologically whenever possible. All efforts should be made to rule out malignancy, but we would suggest adopting management strategies that avoid wide surgical resections in patients where the suspicion of AIP and IgG4-RD is high.
FundingThe authors have no sources of funding to declare.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Federico Baenas D, Soledad Miretti V, Caeiro F, Paira S. Diagnóstico diferencial entre compromiso pancreático en enfermedad relacionada con IgG4 y cáncer de páncreas. Gastroenterol Hepatol. 2021;44:144–155.