Capsule endoscopy was approved by the FDA in 2001. Gastrointestinal bleeding and inflammatory bowel disease are the main indications. It has been available in our hospital since 2004.
MethodsWe retrospectively analysed data from patients who underwent small bowel capsule endoscopy in our hospital from October 2004 to April 2015. Indications were divided into: Obscure gastrointestinal bleeding (occult and overt), inflammatory bowel disease, and other indications. Findings were divided into: Vascular lesions, inflammatory lesions, other lesions, normal studies, and inconclusive studies.
ResultsA total of 1027 out of 1291 small bowel studies were included. Mean patient age was 56.45 years; 471 were men and 556 women. The most common lesion observed was angiectasia, as an isolated finding or associated with other lesions. Findings were significant in up to 80% of studies when the indication was gastrointestinal bleeding, but in only 50% of studies in inflammatory bowel disease. Diagnostic yield was low in the group “other indications”. No major complications were reported.
DiscussionSmall bowel capsule endoscopy has high diagnostic yield in patients with gastrointestinal bleeding, but yield is lower in patients with inflammatory bowel disease.
ConclusionsOur experience shows that capsule endoscopy is a safe and useful tool for the diagnosis of small bowel disease. The diagnostic yield of the technique in inflammatory bowel disease must be improved.
El uso de la enteroscopia con videocápsula fue aprobado por la FDA en 2001. La hemorragia digestiva y la enfermedad inflamatoria intestinal son sus principales indicaciones. En nuestro centro se realiza desde 2004.
Material y métodosHemos recogido de forma retrospectiva los pacientes tratados mediante cápsula de intestino delgado desde octubre de 2004 hasta abril de 2015. Las indicaciones se han dividido en grupos: hemorragia digestiva de origen oscuro, tanto oculta como manifiesta; enfermedad inflamatoria intestinal; otras indicaciones. Los hallazgos se han dividido: lesiones vasculares; lesiones inflamatorias; otras lesiones; estudios normales; estudios no concluyentes.
ResultadosDe un total de 1.291 estudios se ha incluido 1.027 en el análisis. La edad media es 56,45 años, con 471 hombres y 556 mujeres. La enfermedad más frecuentemente observada fueron las lesiones vasculares, asociadas o no a otras lesiones. Cuando la indicación era una hemorragia digestiva, el impacto diagnóstico fue del 80%. En la enfermedad inflamatoria esta cifra solo alcanza el 50%. El rendimiento diagnóstico es mucho menor en el grupo de «otras indicaciones». No se han registrado complicaciones mayores.
DiscusiónLa cápsula de intestino delgado tiene un alto rendimiento diagnóstico en los casos de hemorragia digestiva; el número de estudios con hallazgos positivos es menor en los de enfermedad inflamatoria intestinal.
ConclusionesSe trata de una modalidad diagnóstica segura y de gran utilidad para el diagnóstico de enfermedad del intestino delgado, aunque se precisa mejorar el índice de sospecha en la enfermedad inflamatoria intestinal.
The first mention of capsule endoscopy (CE) in the scientific literature appears in May 2000 in the journal Nature.1 In this short article, Iddan described the development of an ingestible wireless capsule capable of capturing images of the gastrointestinal (GI) mucosa while propelled by peristalsis through the gastrointestinal tract, and communicated the early experiences of his group in testing this device in humans Before long, the first studies were published evaluating the safety and efficacy of the procedure, and comparing it with other techniques (endoscopic and radiological) for the diagnosis of small bowel (SB) pathology.2–5
From the start, the most important and widely used indication for CE studies has been obscure gastrointestinal bleeding (OGIB); the first series were published between 2002 and 2004.5–10 Other, less common indications have also been established for CE, including inflammatory bowel disease (IBD), neoplastic disease, or coeliac disease.11–14 These indications and their corresponding recommendations have been included in clinical practice guidelines (and updates) and consensus documents issued by scientific societies such as the European Society of Gastrointestinal Endoscopy (ESGE), the American Society of Gastrointestinal Endoscopy (ASGE) or the European Crohn's and Colitis Organisation (ECCO).15–20
In 2006, video capsule endoscopy took a giant leap forward with the introduction of colon capsule endoscopy (CCE). The Eliakim group published a series of 91 patients in which the usefulness and safety of this new device in detecting colonic disease was communicated.21 This technology was initially recommended for patients with previous incomplete colonoscopy, or those that were contraindicated or poor candidates for conventional endoscopy.22 However, the number of potential indications for CCE has since been extended, and studies in the benefits of the technique in colorectal cancer screening, follow up of patient with IBD, and even in paediatric patients 23–26, have been published.23–26 To date, one of the last frontiers for video capsule studies is the possibility of exploring the entire gastrointestinal tract, or at least the small and large bowel, with a single device in a single procedure.27
The first SB CE study in our hospital was performed in 2004, making ours one of the first centres to incorporate this technology in Spain. During the early years, a very limited number of procedures were performed, mostly in patients with OGIB.
Since then, however, the number has increased exponentially. Initially, studies in patients with suspected Crohn's disease (CD) were rare, but this indication has increased considerably since 2007, and currently accounts for about half of the procedures performed in our department. CCE was only recently introduced in our hospital, and therefore our experience with this technique is limited and is not included in this study.
We present a descriptive analysis of our experience to date with video capsule enteroscopy since its introduction in our hospital in 2004.
Material and methodsData from all patients diagnosed by SB CE in our hospital between October 2004 and April 2015 were collected retrospectively, including demographics, indications for the study, findings and complications. All records containing incomplete or misleading information were excluded.
IndicationsThe indications for CE studies have been divided into 4 groups:
- -
Occult OGIB: defined as iron deficiency anaemia caused by insensible GI blood loss, the source of which cannot be identified by either upper or lower conventional endoscopy.
- -
Overt OGIB: acute gastrointestinal bleeding manifested as rectal bleeding, haematochezia or melena, the source of which cannot be identified by either upper or lower endoscopy.
- -
IBD: including patients with suspected small bowel Crohn's disease (SBCD) and extension studies, although these latter cases are less frequent than would be expected in our records. In cases of suspected CD, patients had previously undergone colonoscopy with no pathological findings. Although in most of these cases the terminal ileum was visualised and pathology observed, in others the endoscope could not advance beyond the ileocecal valve, and in some the endoscopy report makes no mention of an exploration of the ileum or of a failed attempt to do so.
- -
Other indications: this group includes other, less frequent indications, such as follow-up study of intestinal polyposis, coeliac disease (both initial diagnosis and complications), radiographic findings, nonspecific abdominal pain, etc.
For diagnostic purposes, CE study findings have also been divided into several groups:
- -
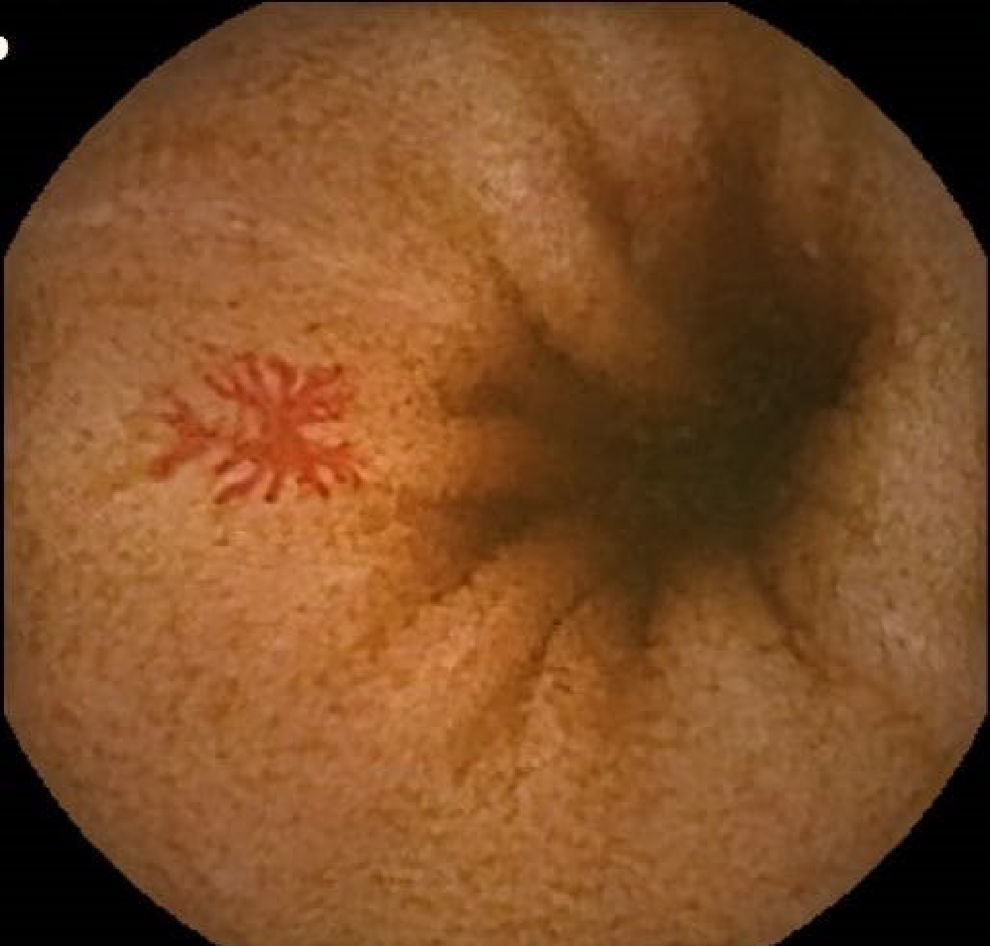
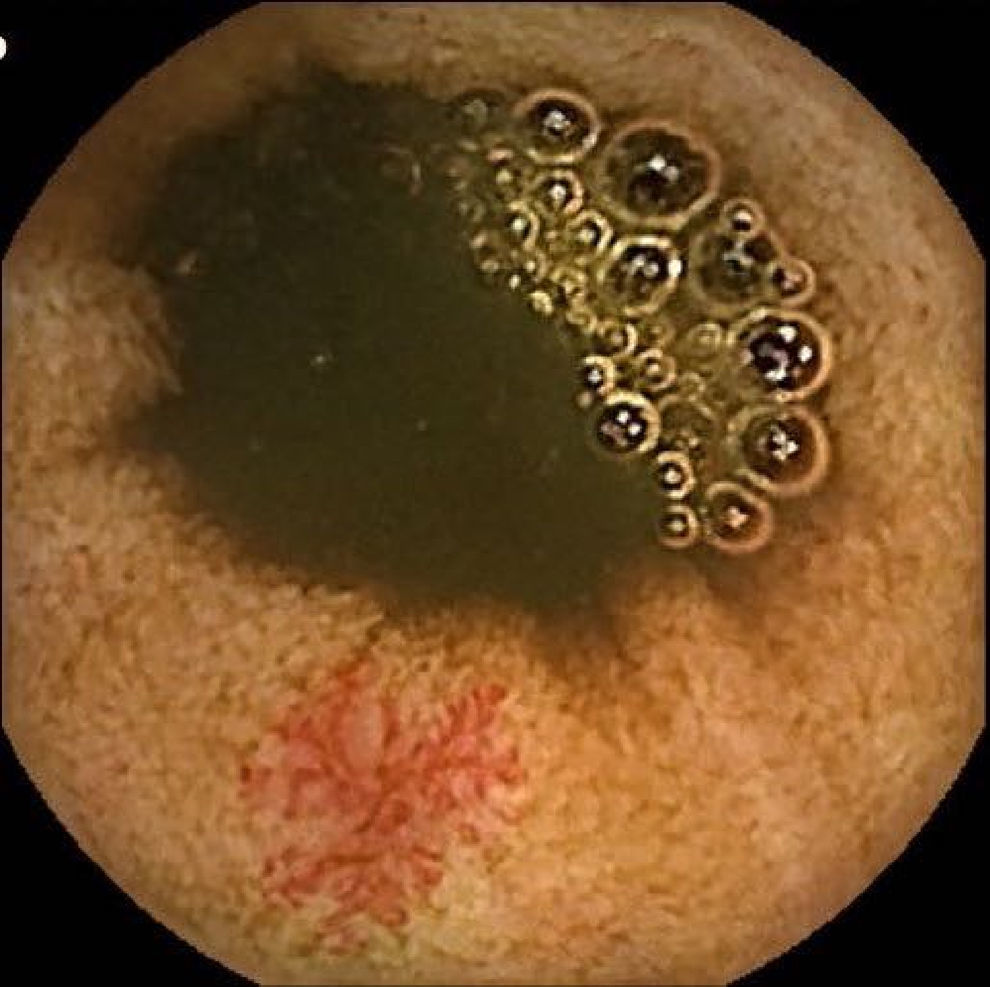
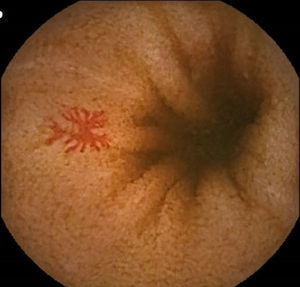
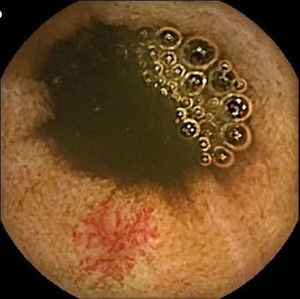
Vascular lesions (VL): mainly angioectasias (Figs. 1 and 2), although patients with other, less common, conditions have also been included, such as intestinal varices.
- -
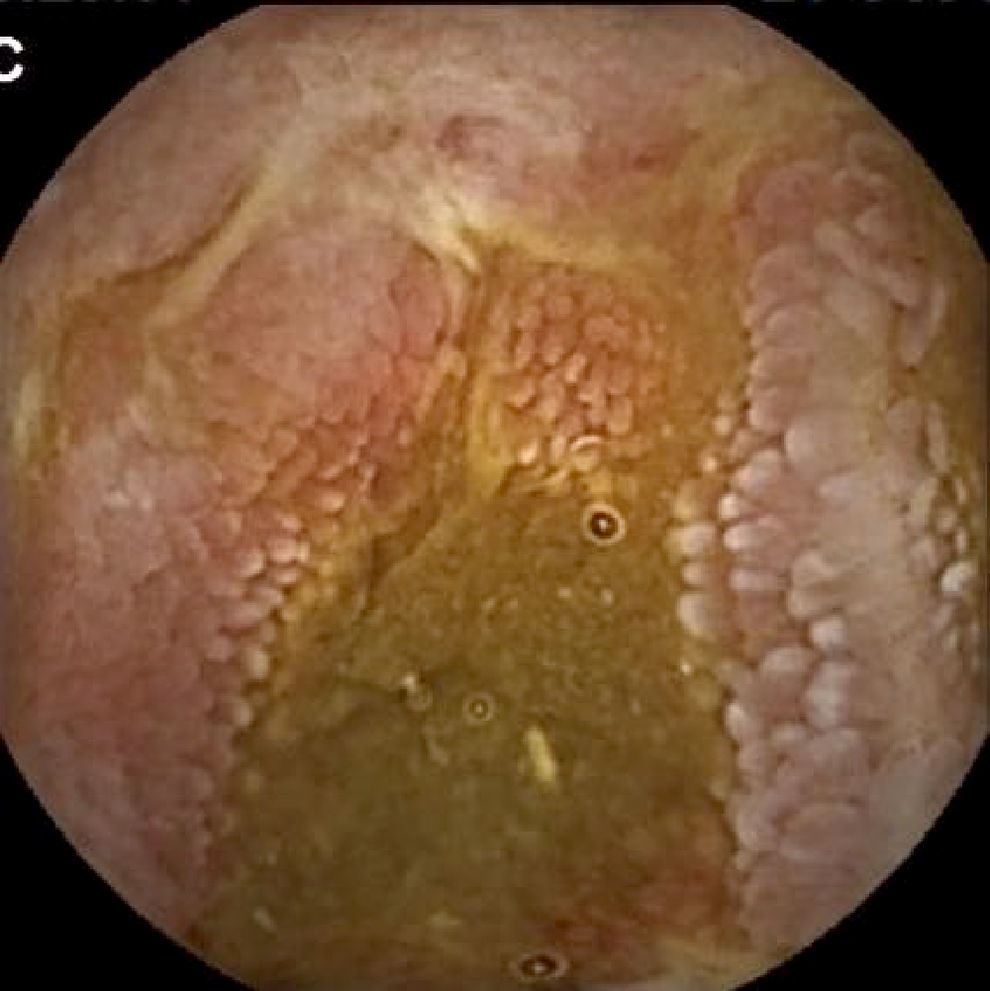
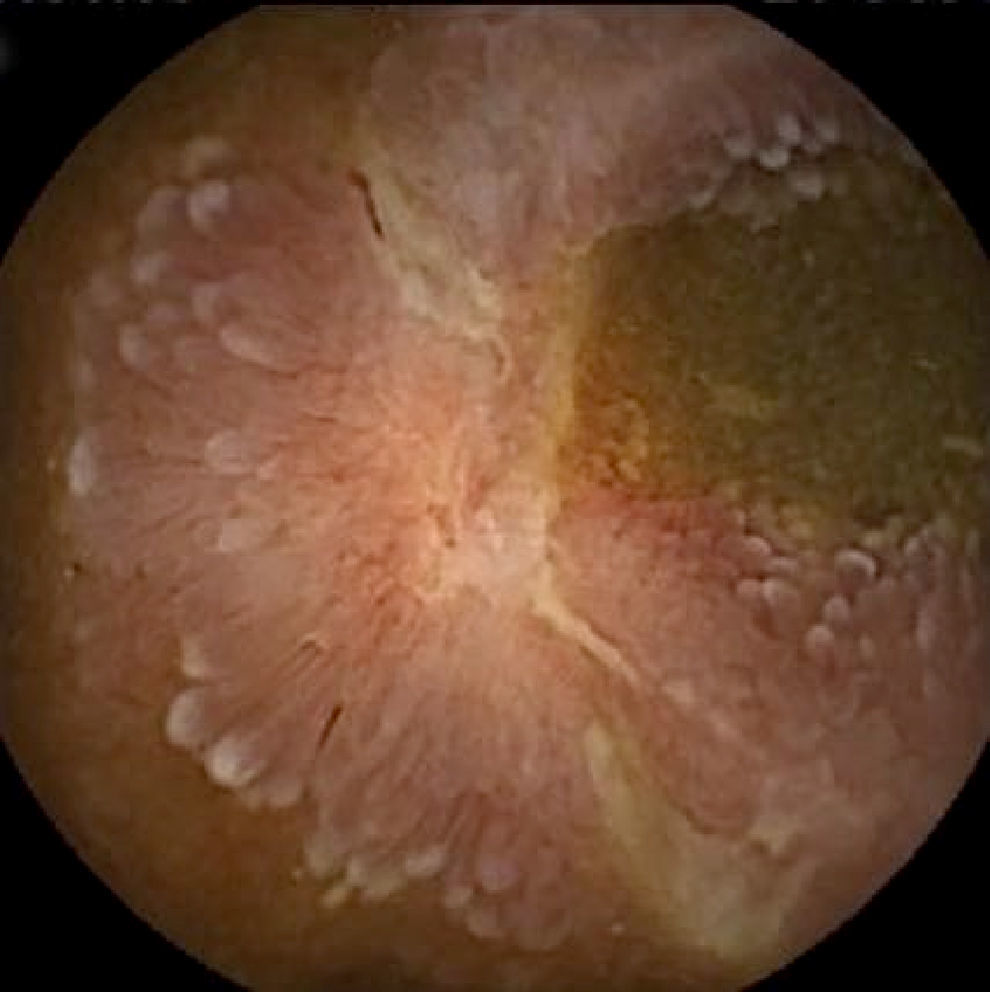
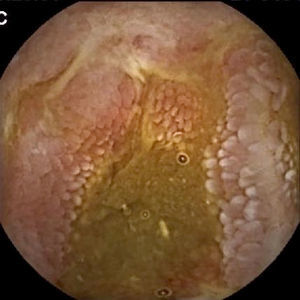
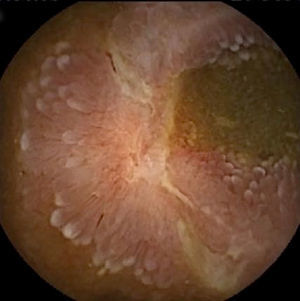
Inflammatory lesions (IL): all findings compatible with SBCD (villous oedema and erythematous mucosa, erosions or ulcers, stenosis, etc.) were included (Figs. 3 and 4). This group also includes erosive lesions that could be related to nonsteroidal anti-inflammatory drug (NSAID) use.
- -
Other lesions: villous atrophy, polyps, tumours (mucosal or submucosal), etc.
- -
Studies with normal findings: all CE scans with no lesions, or with minor lesions that the reader has considered insignificant or incidental in the clinical context of the patient.
- -
Inconclusive studies: studies that were not completed due to gastric or bowel capsule retention, those with technical problems in uploading or processing the images, or poor intestinal cleanliness preventing evaluation of any section of the bowel mucosa, etc.
In all cases, CE was performed using Given Imaging Ltd. (now Medtronic) devices. Over the years, we have used various different video capsules and software supplied by this company, from the M2A® (first capsule sold) to the latest generation, the SB3®.
As of yet, there is no consensus on the best bowel preparation for patients scheduled for SB CE. Some patients are simple instructed to follow a clear liquid diet, while others are given different doses of purgative solutions, sometimes combined with prokinetics or antifoaming agents. Some authors have found no difference between the different preparation strategies,28 while others found the best approach to be preparations containing polyethylene glycol.29 In our case, although a polyethylene glycol preparation is sometimes used, patients scheduled for SB CE are instructed to follow a fibre-free diet in the days leading up to the study, and to fast the night before. Bedridden patients or those with suspected motility disorders are given half a dose of purgative solution.
Using the information collected, we first performed a descriptive analysis, taking into account the demographic characteristics of the patients in each indication and the frequency of each finding. Following that, we determined the frequency of each endoscopic finding in each indication and the percentage of studies with normal findings in order to evaluate the diagnostic yield of the technique in each case.
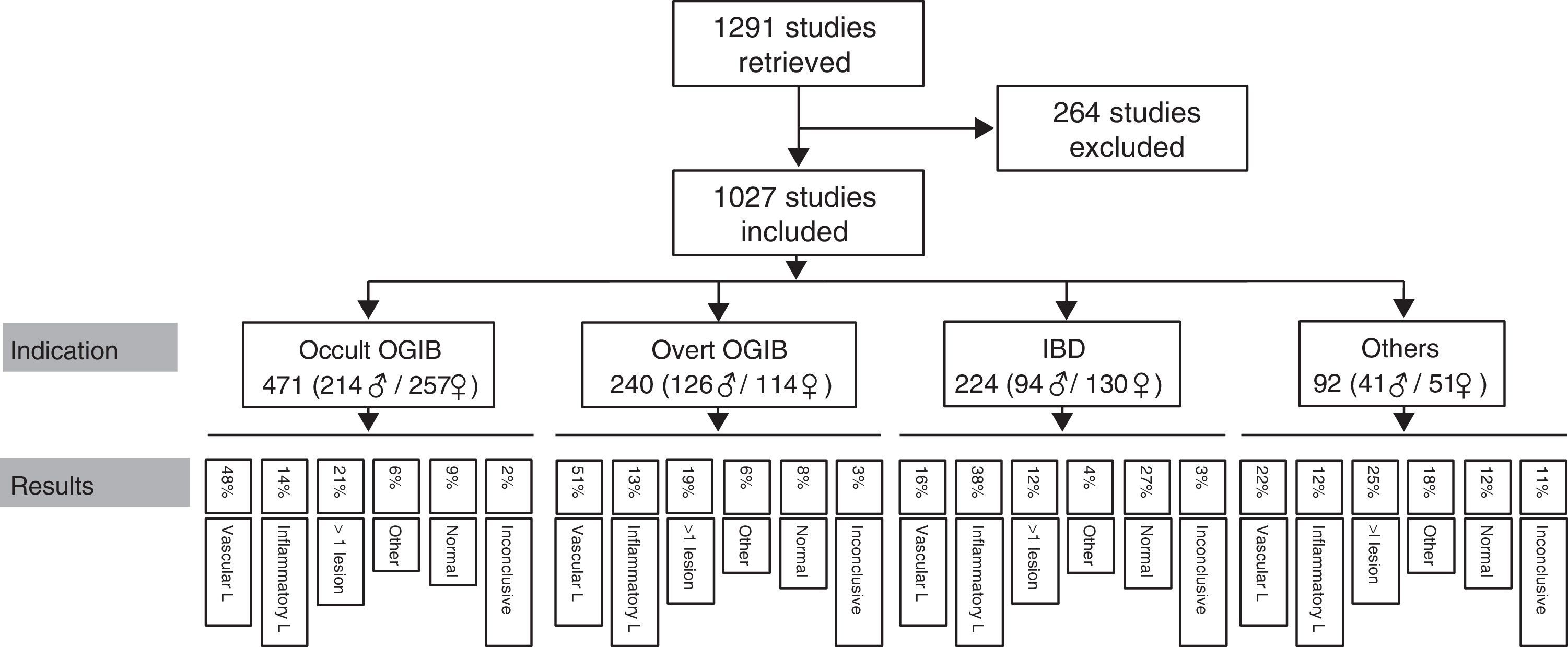
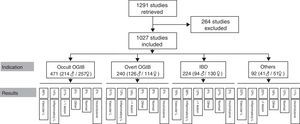
ResultsA total of 1291 procedures were documented in our CE unit from October 2004 to April 2015. Of these, 264 were excluded from the analysis due to incomplete or misleading information, or because the record was not available. This left a total of 1027 CE studies finally included in the analysis.
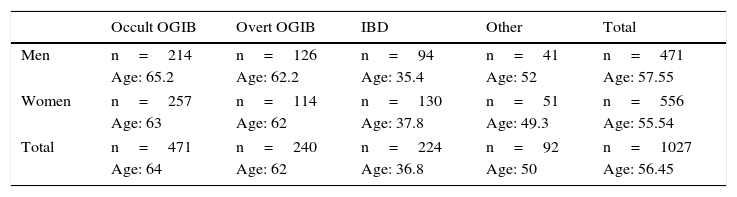
Patients had a mean age of 56.45 years (4–91 years), with a total of 471 men (mean age 57.55 years) and 556 women (mean age 55.54 years). The most common indication is obscure OGIB (n=471; 214 men/257 women), followed by overt OGIB (n=240; 126 men/114 women), IBD (n=224; 94 men/130 women) and “other indication” (n=92; 41 men/51 women). Table 1 shows the distribution of the various indications by sex and age.
CE indications according to the mean age and sex of patients.
| Occult OGIB | Overt OGIB | IBD | Other | Total | |
|---|---|---|---|---|---|
| Men | n=214 | n=126 | n=94 | n=41 | n=471 |
| Age: 65.2 | Age: 62.2 | Age: 35.4 | Age: 52 | Age: 57.55 | |
| Women | n=257 | n=114 | n=130 | n=51 | n=556 |
| Age: 63 | Age: 62 | Age: 37.8 | Age: 49.3 | Age: 55.54 | |
| Total | n=471 | n=240 | n=224 | n=92 | n=1027 |
| Age: 64 | Age: 62 | Age: 36.8 | Age: 50 | Age: 56.45 | |
IBD: irritable bowel disease; OGIB: obscure gastrointestinal bleeding.
In the global analysis of findings in EC studies, the most frequently observed was VL, in 583 cases (57% of included studies), either as the only finding or associated with other lesions. IL was found in 329 cases (32% of all studies included), as a single lesion or in association with other lesions. Other lesions, primarily polyps or submucosal tumours, were found in 159 cases (15% of included studies), most of them as secondary or incidental findings.
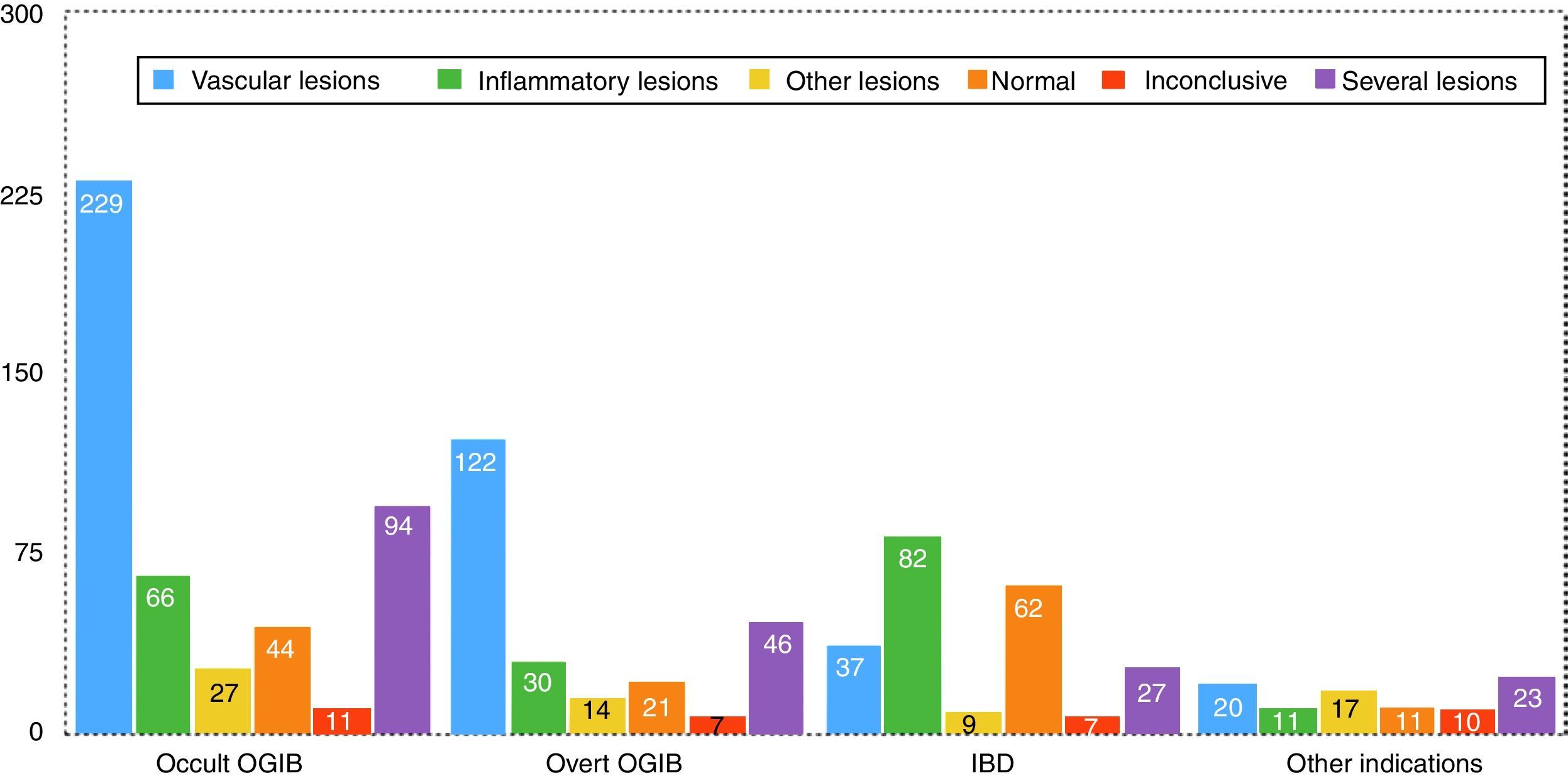
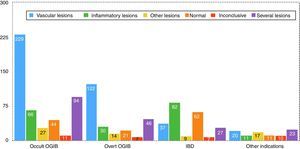
Findings by indication are as follows (these data are also summarised in Figs. 5 and 6). Among the 471 cases of occult OGIB, VL was found in 229 (48%), 66 patients (14%) presented IL (mostly patients treated with NSAIDs, although the group also included several patients diagnosed with CD in whom the cardinal symptom was anaemia), in 97 (21%) more than one pathology was found (most frequently VL plus IL in patients on antithrombotic treatment), in 27 cases (6%) incidental findings were reported, and in 44 and 11 patients (9% and 2%) the studies were normal or inconclusive, respectively. Among the 240 patients presenting overt OGIB, 122 (51%) were diagnosed with VL, 30 (13%) with IL, 46 (19%) presented several lesions (as in the case of occult OGIB, most frequently VL plus IL), incidental findings were reported in 14 (6%), and in 21 and 7 (8% and 3%) cases, the studies were normal or inconclusive, respectively. Among the 224 patients with an indication of IBD, 82 presented IL (38%), VL was found in 37 (16%) cases (probably an incidental finding in the context of patients with suspected IBD), in 9 studies (4%) polyps or submucosal tumours were found, 27 (12%) patient presented more than one pathology (again, in most cases VL plus IL), and 62 and 7 (27% and 3%) cases, the studies were normal or inconclusive, respectively. Among the 92 patients in the “other indications” group, VL was found in 20 (22%), IL in 11 (12%), 17 presented polypoid or submucosal lesions (18%), 23 (25%) presented various types of injury, and in 11 and 10 (12% and 11%) cases, the studies were normal or inconclusive, respectively. Notable in this group is the diagnosis of one case of ulcerative jejunitis and 3 cases of intestinal lymphoma in coeliac patients.
Overall, the findings in up to 80% of OGIB cases (mainly VL, IL or both) justified the indication. In the case of patients with suspected IBD, the indication was justified in around 50%. The remaining studies were inconclusive, or no pathology or incidental lesions were found. The diagnostic yield of the “other indications” group is far lower, with many incidental findings and only 21% of findings related to the indication.
No major complications were noted in any of the records analysed. Regarding minor complications, several cases of CE retention at various levels of the digestive tract were documented. Four cases of transient gastric EC retention were found. In all these cases, the capsule's battery ran out before it left the gastric cavity, but endoscopic removal was not required because in all patients it ultimately passed through the small bowel. Five cases of EC retention in the SB were also reported: a 65 year-old woman with occult OGIB; a 47- year-old man, also with occult OGIB; an 85-year-old inpatient with overt OGIB; a 28-year-old women with suspicion of IBD, and a 19-year-old man, also with suspicion of IBD. All these patients presented SB stenosis in the context of CD (not suspected prior to CE) and were managed conservatively with medical treatment, without the need for emergency surgery. Two of these (the 65-year-old women and 47-year-old man) required surgical treatment for their stenotic inflammatory disease, but in both cases the procedure was elective and the timing was not determined by capsule retention. In this regard, it is interesting to note that in our unit we have been using the Agile® Patency degradable capsule since early 2013 in cases where SB stenosis is suspected. Prior to this, we used radiological techniques, such as barium transit, although there was no evidence that this prevented CE retention, and the practice was abandoned. Since that time, 27 degradable capsules have been administered: in 5 cases CE administration was contraindicated due to failure to excrete the capsule; in the remaining 22 cases in which the degradable capsule was excreted intact, CE was subsequently administered without any complications. In the foregoing cases of CE retention, SB stenosis was not previously suspected and no prior permeability test had been performed.
CE has been administered in patients with cardiac devices, with no cases of malfunction reported. In our experience, we found that administering CE in the vicinity of hospital area equipped with telemetry equipment (cardiology ward or intensive care unit) can interfere in the transmission of images from the capsule to the recorder.
DiscussionSince its introduction, CE has proved to be a useful tool for the diagnosis of SB pathology. The most common indication for CE, and the one with the highest diagnostic yield, is OGIB (except in children, when the most frequent indication is IBD30). There have been many studies and reviews on this subject, with diagnostic efficacy in cases of OGIB ranging from 47% to 84%. However, in many cases the indications are heterogeneous and the timing of the CE varies considerably, making it difficult to compare the findings.31–34 Vascular lesions (equivalent to VL in our analysis), particularly angioectasias, are the most common pathology found in this group of patients, followed by inflammatory lesions (equivalent to IL in our series) either in the context of SBCD or NSAID use.9,10,35,36 In this respect, the results of our retrospective series do not differ from what has been previously reported in the literature: OGIB is the most common indication and the one with the highest diagnostic yield (in both occult and overt OGIB, lesions that would justify the indication were found in around 80% of cases), with VL being the most common finding.
It is interesting to note the frequency of VL in indications other than OGIB, particularly in elderly patients. The incidental finding of VL in all groups could suggest that in patients with OGIB, the presence of some of these lesions is fortuitous, and they are not the cause of their anaemia. However, we cannot confirm this hypothesis or make any general recommendations in this regard. Although systems for assessing and categorising the bleeding risk of different lesions observed during CE have been put forward,37 is not always easy to determine the clinical relevance of these when reading CE images.
The reason why the use of CE in patients with OGIB is particularly cost effective in terms of diagnostic yield is because several studies and systematic reviews have defined the factors that predict the likelihood of detecting pathology in these patients. This allows clinicians to select the patients who can most benefit from the technique on the basis of highly specific indications, which is particularly relevant considering the cost of these procedures in terms of both the worktime of professionals and resources.38 The factors predicting diagnostic yield include acute bleeding, need for oral iron therapy or blood products, being male, age over 65 years, use of NSAID or antithrombotic therapy, liver disease, etc.39–42 In IBD, however, fewer factors predicting diagnostic yield in CE have been defined, and thus the percentage of positive findings in this indication is lower. CE has shown its usefulness in the diagnosis of CD43 and its superiority over radiological techniques,11,12 and a consensus conference (ICCE-International Conference on Capsule Endoscopy) held in 2005 established the role of the technique in patients with suspected SBCD but normal findings in other studies.44 Shortly thereafter, the conclusions of this panel of experts laid the basis for a consensus statement with several recommendations for CE,45 including a suite of criteria on which to base suspicion of SBCD:
- -
Clinical criteria: abdominal pain, chronic diarrhoea, weight loss, growth retardation.
- -
Extraintestinal manifestations: arthropathy, pyoderma, perianal disease, primary sclerosing cholangitis.
- -
Analytical markers: anaemia, elevated C-reactive protein or erythrocyte sedimentation rate, leukocytosis, serological markers, faecal markers.
- -
Imaging study anomalies: SB barium transit, computed tomography.
Subsequent evidence has shown that patients meeting these criteria are more likely to present lesions in CE studies, and the greater the number of criteria met, the higher the diagnostic yield.46 Only a few studies have explored the relationship between different symptoms or laboratory parameters (mostly in isolation) and SBCD findings; they are, moreover, heterogeneous, sometimes difficult to compare, and with inconsistent results.47–50 Some, albeit small, series have analysed the association between different criteria for suspicion, and have concluded that symptomatic patients with one or more biochemical markers51 or patients presenting anaemia with thrombocytosis52 are the best candidates for CE to confirm suspicion of SBCD. In our series, inflammatory lesions were found in fewer than half of patients undergoing CE due to suspected IBD. CE has a particularly high negative predictive value53 for ruling out SBCD, and is an excellent tool for this purpose. This highlights the impact of negative diagnostic results on patient management; however, the technique is time consuming and costly,38 and therefore candidates must be carefully selected.
Although the most important indications for CE are OGIB and IBD, the technique has also been useful in difficult to diagnose or refractory coeliac disease, tumours, SB polyposis monitoring, abdominal pain, etc.54–57 The latest update of the European guidelines on CE and device-assisted enteroscopy for the diagnosis and treatment of small-bowel disorders58 accepts the use of CE in the management of patients with suspected coeliac disease who refuse or are contraindicated for conventional endoscopy, or in cases of refractory coeliac disease. The authors, however, do not recommend CE as a routine diagnostic procedure for this disease, but do recommend it for monitoring patients with polyposis syndromes and in those with suspected SB cancer. The guidelines are silent on the potential use of CE in non-specific abdominal pain, although some studies have reported a diagnostic yield of approximately 21% in these cases.59 In our series, very few CE studies were performed for these indications (n=92), and the findings were heterogeneous. The most frequent indication, and the least justified in terms of diagnostic findings, is non-specific chronic abdominal pain. In contrast, the greatest number of CEs with diagnostic findings directly related to the indication were observed in suspected refractory or complicated coeliac disease.
Complication rates reported in the literature are exceptionally low. These, associated with technical problems and incomplete scans, lead to diagnostic failure in some studies.60 The most important complication is gastric or SB capsule retention, generally in relation to stenosis caused by Crohn's disease. The ICCE defined CE retention as the presence of the capsule in the digestive tract for more than 2 weeks61; it is usually asymptomatic, and cases of impaction leading to acute SB obstructing are extremely rare. Retention usually responds to medical or conservative treatment, and endoscopic or surgical removal is rarely required.60,61 In our series, we observed very few complications: 9 cases of capsule non-progression (4 cases where the battery ran out before leaving the gastric cavity, and 5 cases of SB retention). Only 2 patients required surgery; in both cases this was an elective intervention for stenosis refractory to medical treatment, and the timing of the procedure was not determined by capsule retention.
Although the topic is widely debated, the presence of a pacemaker or defibrillator is not currently a contraindication for CE.62 Many of the patients in our series had cardiac devices, and no cases of malfunction were reported. We did find evidence, however, that performing CE in the vicinity of telemetry systems can cause interference in the transmission of images to the recorder, resulting in multiple gaps in nearly all the videos downloaded from patients located near such equipment. Therefore, we avoid administering the capsule to patients hospitalized in cardiology wards or in intensive care units, and prefer to postpone CE until they can be moved to another area, provided this is feasible and the study is not urgent.
We collected data from patients between the ages of 4 and 91 years. Many studies have confirmed the safety and usefulness of the technique in elderly patients.63,64 Less evidence is available in children, although the few studies published in this context have confirmed the safety and high diagnostic yield of CE in the paediatric population.65,66 We obtained data from a total of 42 patients 16 years of age or younger, including 18 children 12 years of age or younger, and found no reports of complications in this age group.
ConclusionsCapsule endoscopy has proven to be a useful technique for the diagnosis of SB disease, and it is now widely used in the management of patients with chronic anaemia due to GI blood loss and IBD.
CE has a high diagnostic yield in patients with both occult and overt OGIB. This is largely due to the studies and reviews that have defined the factors that predict the likelihood of detecting SB lesions in these patients. The same cannot be said of IBD: a better definition of clinical and analytical factors that can increase the likelihood pathological findings in CE is necessary in this indication.
Aside from OGIB and IBD, CE can be useful in a wide range of indications, albeit with a far lower diagnostic yield. Careful patient selection is needed to ensure the cost-effectiveness of this technique in these populations.
Generally speaking, CE is a safe procedure, and the few complications associated with this technique can usually be managed conservatively, with little need for invasive procedures. In our experience, capsule endoscopy is also safe in children and in the elderly.
Our series has the limitation of being a retrospective study performed in a single centre. Although the final sample size is large, many patients were excluded due to lack of information, either because the CE studies date from several years ago, or because they were performed in patients referred from neighbouring hospitals, and the reports are no longer available. Either way, this is a limitation for our study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Egea Valenzuela J, Carrilero Zaragoza G, Iglesias Jorquera E, Tomás Pujante P, Alberca de las Parras F, Carballo Álvarez F. Análisis histórico de la experiencia en cápsula endoscópica de intestino delgado en un hospital terciario español. Gastroenterol Hepatol. 2017;40:70–79.