There is limited information regarding the impact of patients’ perception of injection pain on adherence to treatments, specifically in inflammatory bowel disease (IBD) patients. Therefore, we aimed to determine the impact of the pain associated with the subcutaneous administration of adalimumab in patients with IBD treated with the old formulation and the new low-volume/citrate-free formulation.
MethodsA specifically-designed questionnaire was completed by 76 patients with IBD, who started treatment with adalimumab before the availability of the low-volume/citrate-free formulation and were switched to this new formulation. Intensity of pain was measured by using visual analog scales (VAS).
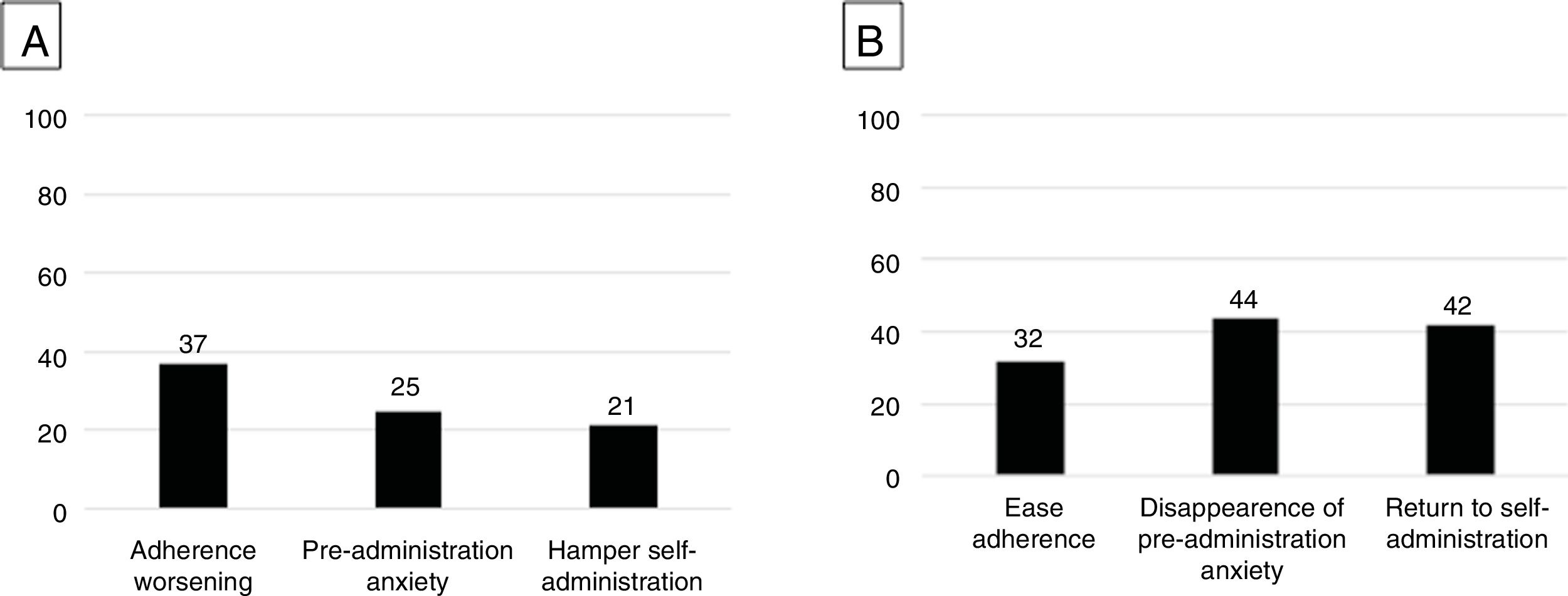
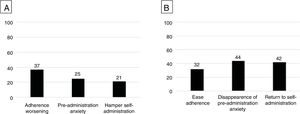
ResultsA total of 62 patients (82%) experienced injection-related pain with the initial formulation. The perception of pain was associated with a decreased adherence to the treatment (37%), an increase in pre-administration anxiety (25%) or, as a consequence, the patient required someone else to carry out the injection (21%). Younger age was the only factor associated with pain perception. After switching to the new formulation, perception of pain persisted only in 2 patients (3%). Among those who felt pain with the initial formulation, pre-administration anxiety disappeared in 44%; 32% and 42% stated that the new formulation eased adherence and self-administration.
ConclusionsThe perception of pain related to the subcutaneous administration of therapy negatively impacts on treatment adherence in IBD patients. Improved formulations for subcutaneous administration of drugs can positively impact patients’ convenience and adherence.
La información sobre el impacto de la percepción del dolor asociada a inyección sobre la adherencia a los tratamientos subcutáneos es limitada específicamente en pacientes con enfermedad inflamatoria intestinal (EII). Nuestro objetivo fue determinar el impacto del dolor asociado con la administración subcutánea de adalimumab en pacientes con EII y tratados con la fórmula antigua y la nueva de bajo volumen/sin citrato.
Materiales y métodosSetenta y seis pacientes con EII que iniciaron tratamiento con adalimumab antes de la disponibilidad de la formulación de bajo volumen/sin citrato y se cambió a esta nueva formulación completaron un cuestionario diseñado específicamente. La intensidad del dolor se midió mediante escalas visuales analógicas (EVA).
ResultadosSesenta y dos pacientes (82%) experimentaron dolor relacionado con la inyección con la formulación inicial. La percepción de dolor se asoció con menor adherencia al tratamiento (37%), aumento de la ansiedad pre-administración (25%) o, como consecuencia, el paciente rehuyó la autoinyección (21%). La edad más joven fue el único factor asociado a la percepción del dolor. Tras cambiar a la nueva formulación, la percepción del dolor persistió solo en 2 pacientes (3%). Entre los que referían dolor con la formulación inicial, la ansiedad pre-administración desapareció en el 44%; el 32 y 42% afirmaron que la nueva formulación facilitaba la adherencia y la autoadministración.
ConclusionesLa percepción del dolor relacionada con la administración subcutánea de fármacos tiene un impacto negativo en la adherencia en pacientes con EII. Las formulaciones optimizadas de administración subcutánea pueden tener un impacto positivo en la comodidad y adherencia.
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is a chronic condition that produces inflammation of the gastrointestinal tract1,2 and it is becoming highly prevalent among Western countries. Main therapeutic goals include to induce and maintain the clinical remission and, ultimately, restore the quality of life of patients.3 Conventional IBD management involved drug therapy (corticosteroids, aminosalicylates, and immunomodulators) and, eventually, surgery.4,5 Anti-tumor necrosis factor (TNF) agents have been shown to be effective in CD and UC patients6,7 and they are usually prescribed in patients with impaired quality of life. International guidelines recommend their use in patients with moderate-to-severe active UC or CD who are intolerant, have contraindications, or responded inadequately to conventional therapies.8–10 The human monoclonal anti-TNF adalimumab (ADA) is administered subcutaneously.11 Injection site reactions (including erythema, itching, hemorrhage, or pain) are common mild-to-moderate side effects reported with medications and proteins. They have been initially documented in patients with rheumatoid arthritis (RA) receiving ADA.12 Needle size and sharpness, injection volume, or inactive ingredients (buffers, for instance) can impact in the pain related to the subcutaneous injection.13–15 Sodium citrate may be one of these ingredients causing discomfort to patients.16
A new improved formulation of ADA was marketed and launched in Spain in January 2017, with no citrate buffer and a reduced volume to be injected. Since then, this citrate-free formulation is the only formulation available.
Non-adherence to treatments is a frequent problem in chronic diseases.17 In IBD, several studies reported rates of non-adherence ranging from 30% to 69%.18,19 To date, there is limited information regarding the impact of injection pain on patients’ experience and adherence to treatments.20,21 To our knowledge, most available studies were performed with patients with RA, but no study has been specifically addressed to IBD patients. Therefore, our aims were to determine the impact of the pain associated with the subcutaneous administration of ADA in patients with IBD, and to evaluate the switch to the novel low-volume/citrate-free formulation.
Materials and methodsThe study was performed from June to September 2017 in 2 referral IBD units in the Barcelona area (Hospital Santa Creu i Sant Pau and Hospital Universitari Germans Trias i Pujol). Patients were identified from local IBD databases to participate in the study. Both hospitals have similar number of patients receiving treatment with ADA and followed-up. Similarly, the new low-volume/citrate-free formulation of ADA was introduced in January 2017 in both hospitals. Inclusion criteria were as follows: (1) patients with diagnosis of CD or UC, receiving active treatment with the innovator ADA (Humira™, AbbVie Biotechnology GmbH, Germany) between June and September 2017, and (2) at least 12 months of continuous treatment with ADA before the inclusion in the study. At the time the study was performed, no biosimilar of ADA was available yet. The completion of the survey was considered as the explicit consent to participate. Therefore, all the patients included in the study completed the survey once they had been treated with both the old and the new formulations of adalimumab.
Eligible patients were invited to complete an anonymous survey designed to evaluate the pain perception and consequences of the new ADA formulation. The invitation was made by postal mail in Hospital Santa Creu i Sant Pau and directly during a routine visit to the specialist in Hospital Universitari Germans Trias i Pujol. The postal surveys were sent to the patients by postal mail including a stamped return envelope. The survey consisted on a structured questionnaire with demographic and basic clinical variables (gender, age, smoking habits, study level, type of IBD) as well as some issues related to ADA therapy (age and year when ADA was started, dosing frequency in the last 6 months, person who usually administered the drug, and experience with other subcutaneous drugs preceding ADA treatment). Patients were asked about the perception of pain related to ADA subcutaneous injection, and its intensity was rated by using a visual analog scale (VAS) ranging from 0 (no pain) to 10 (a lot of pain). Patients were also asked whether the presence of pain was important for treatment compliance, and how the pain affected the treatment (non-adherence, concomitant anxiety, need for someone else to administer the injection). Patients were asked whether the perception of pain changed after switching to the novel formulation. A second VAS aimed to rate the intensity of pain in this period. Similarly, in case of experiencing pain with the novel formulation, patients were asked if the presence of pain was important for continuing with the treatment, and how the pain affected the treatment. Patients also rated the change to the novel formulation by using a VAS (from 0 – no change/satisfaction to 10 – complete change/satisfaction), and informed which aspects improved (anxiety, adherence, self-administration).
Statistical analysisCategorical variables are expressed as absolute and relative frequencies, and continuous variables as the median and interquartile range (IQR). Association between injection pain and demographic and clinical characteristics of patients were analyzed by using the Fisher Exact or t tests, when required. Statistical significance was established with p<0.05.
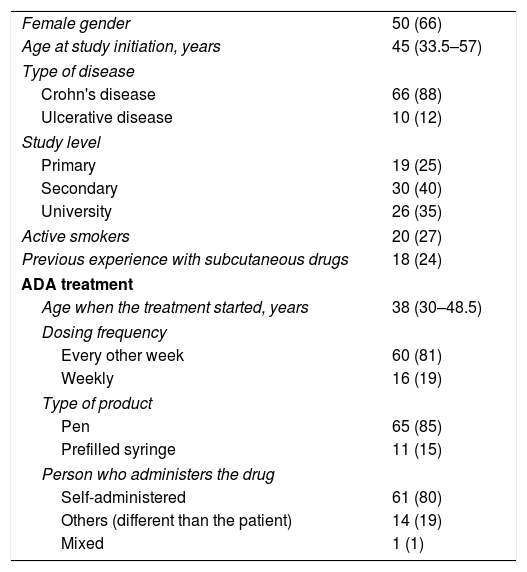
ResultsA total of 76 patients participated in the study. All 31 patients who were invited to presentially answer the survey accepted to do so. Among 65 patients who were invited by a postal mail, 45 (69%) returned the fulfilled survey. Demographic and clinical characteristics of subjects are shown in Table 1. There was a predominance of females, non-smokers, and CD patients. Only one quarter had prior experience with subcutaneous drugs. ADA therapy was mostly self-administered every other week by means of a pen device.
Demographic and clinical characteristics of patients. Data are expressed as raw data (frequency) or median (interquartile range).
| Female gender | 50 (66) |
| Age at study initiation, years | 45 (33.5–57) |
| Type of disease | |
| Crohn's disease | 66 (88) |
| Ulcerative disease | 10 (12) |
| Study level | |
| Primary | 19 (25) |
| Secondary | 30 (40) |
| University | 26 (35) |
| Active smokers | 20 (27) |
| Previous experience with subcutaneous drugs | 18 (24) |
| ADA treatment | |
| Age when the treatment started, years | 38 (30–48.5) |
| Dosing frequency | |
| Every other week | 60 (81) |
| Weekly | 16 (19) |
| Type of product | |
| Pen | 65 (85) |
| Prefilled syringe | 11 (15) |
| Person who administers the drug | |
| Self-administered | 61 (80) |
| Others (different than the patient) | 14 (19) |
| Mixed | 1 (1) |
A total of 62 patients (82%) experienced pain during the subcutaneous injection with the former formulation, with a mean subjective intensity of 7 (IQR, 4–8.1) on the VAS. The perception of pain was associated with a decreased adherence to the treatment in 37% of cases and pre-administration anxiety in 25%. These patients reported to require someone else for injecting in 21% (Fig. 1A). No clinical or treatment-related factors were associated with the perception of pain. Among demographic variables, only a younger age was associated with pain perception (p<0.001).
(A) Perceived impact of injection-related pain (n=62) on adherence, drug administration related anxiety and need for someone to administer the drug. (B) Perceived impact of improvement/disappearance of injection related pain (n=60) on improving adherence, disappearance of pre-administration anxiety and return to self-administration.
After switching to the novel low-volume/citrate-free formulation, perception of pain persisted only in 2 out of 62 patients (3%); no patients who did not perceive pain with the former formulation did it with the new one. Among those who felt pain with the initial formulation, pre-administration anxiety disappeared in 44%, 32% of them stated that the new formulation improved treatment adherence easier and 42% that made self-administration easier (Fig. 1B). The change to the novel formulation was rated 9.5 (IQR, 8.9–10) in the VAS (in which 10 was complete change/satisfaction).
DiscussionNon-adherence to treatments is a common issue in chronic diseases, and it is associated with the loss of treatment efficacy, poor clinical outcomes, decreased quality of life, and increased medical costs.22–24 Subcutaneous administration may be convenient due to ease of administration and patients’ autonomy. However, injection-related pain might hamper adherence. Bolge et al.20 evaluated the reasons for treatment discontinuation of subcutaneous anti-TNFs in 250 RA patients, with injection site pain or discomfort being reported in 14% and 13% of patients, respectively. Similarly, Curtis et al.25 observed that 22% of patients experienced moderate-to-severe pain at the injection-site in a large series of RA patients receiving injectable anti-TNFs. Borrás-Blasco et al.26 focused on improvements of ADA formulation and evaluated the impact of switching the prefilled syringe of ADA as compared to the self-injection pen in 55 patients; self-administration increased from 51% to 84% and the perceived pain was significantly reduced. Likewise, Nash et al.21 compared the injection pain between an original ADA formulation and a low-volume/citrate-free one in 125 patients with RA in two international randomized studies. Both studies demonstrated a significant decrease of pain, with a mean pooled VAS value of −2.5. Our results in IBD patients are in agreement with these studies in showing that the low-volume/citrate-free formulation improved markedly injection-related perception of pain. Beyond this, one of the goals of our study was to evaluate the relationship of injection pain perception with treatment adherence and patients’ experiences. More than one third of our patients indicated that the pain affected the treatment adherence; however, this was widely reverted when switching to the new formulation.
Finally, younger age was the only factor significantly associated with the perception of pain at the injection site. A recent study from Matsui et al.27 found an inverse correlation between the incidence of injection site reactions and age. The authors pointed out to a differential sensitivity to medication between younger and older patients for explaining this finding.
The present study has some limitations. Firstly, the survey was performed once the patients did not use for many months de former formulation. Of course, this might influence the past perception of pain, mostly by its overestimation. Second, we assessed adherence by direct question and did not use validated tools to assess adherence; however, this might underestimate the impact of pain perception on adherence and, therefore, would underestimate the relevance of pain reduction on adherence.
ConclusionsIn conclusion, pain is a side effect associated with the subcutaneous administration of drugs that should be carefully taken into account as it may negatively impact on treatment adherence and, ultimately, on the quality of life of patients. Improved formulations for subcutaneous administration of drugs can impact deeply and positively on patients’ convenience.
Authors’ contributionsCG, and EG-P study and survey designs, data collection and analysis, writing up the first draft of the paper. ED study and survey designs, data analysis, and writing up the first draft of the paper. JG review survey, data collection and analysis, critical review the paper. LM, MM, FB, FC, CG-M and MC review survey, data collection, critical review the paper.
Conflicts of interestMM has served as a speaker or has received research or education funding from MSD, AbbVie, Takeda, Janssen, Ferring, Pfizer; FC has served as a speaker or has received educational grants from Takeda, Janssen, MSD, and Ferring; MC has served as a speaker for Takeda, Janssen, Faes Farma and MSD; ED has served as a speaker, or has received research or educational funding or advisory fees from MSD, AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Celgene, Adacyte Therapeutics, Otsuka Pharmaceuticals, Ferring, Shire Pharmaceuticals, Tillots Pharma, Thermofisher, Grifols, Gebro.; EG-P has served as a speaker, or has received research or educational funding or advisory fees from MSD, AbbVie, Takeda, Kern Pharma, Pfizer, Janssen, Ferring, Shire Pharmaceuticals, Tillots, Falk, Faes, Gebro; all the remaining authors declared no conflicts of interest.